Pelvic inflammatory disease is a clinical condition resulting from infection of the upper genital tract.
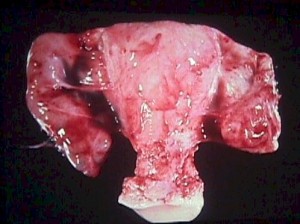
It can be suspected if the patient exhibits:
- cervical motion tenderness
or - uterine tenderness
or - adnexal tenderness.
If the patient also exhibits any of the following, PID becomes likely:
- oral temperature >101°F (>38.3°C);
- abnormal cervical mucopurulent discharge or cervical friability;
- presence of abundant numbers of WBC on saline microscopy of vaginal fluid;
- elevated erythrocyte sedimentation rate;
- elevated C-reactive protein; and
- laboratory documentation of cervical infection with N. gonorrhoeae or C. trachomatis.
From the perspective of operational medicine, there are two subcategories that are important: Mild PID and Severe PID. They are distinguished by the absence of fever.
PID: Mild
These patients typically experience a gradual onset of mild bilateral pelvic pain with purulent vaginal discharge. Temperature does not exceed 100.4 (38.0). They may also complain of lassitude, and headache. Symptoms more often occur shortly after the onset or completion of menses.
Excruciating pain on movement of the cervix and uterus is characteristic of this condition. Pelvic and abdominal tenderness is always bilateral except in the presence of an IUD.
Gram-negative diplococci in cervical discharge or positive chlamydia culture may or may not be present. WBC and ESR are elevated.
These patient usually respond well to simple treatment with bedrest, IM and oral antibiotics (see below). A rapid recovery (days) is expected and if such recovery does not occur, the diagnosis should be reconsidered and therapy changed.
PID: Moderate to Severe
With moderate to severe PID, there is a also a gradual onset of moderate to severe bilateral pelvic pain with purulent vaginal discharge, but with fever >100.4 (38.0), lassitude, and headache. Symptoms more often occur shortly after the onset or completion of menses.
Excruciating pain on movement of the cervix and uterus is characteristic of this condition. Hypoactive bowel sounds, purulent cervical discharge, and abdominal dissension are often present. Pelvic and abdominal tenderness is always bilateral except in the presence of an IUD.
Gram-negative diplococci in cervical discharge or positive chlamydia culture may or may not be present. WBC and ESR are elevated.
Treatment consists of bedrest, IV fluids, IV antibiotics, and NG suction if ileus is present. Since surgery may be required, transfer to a definitive surgical facility should be considered. These patients are very ill, and will not typically respond in a few days. Their recovery is more often measured in weeks to months.
Treatment*
PID treatment regimens should provide empiric, broad-spectrum coverage of likely pathogens. Multiple parenteral and oral antimicrobial regimens have been effective in achieving clinical and microbiologic cure in randomized clinical trials with short-term follow-up. However, only a limited number of studies have assessed and compared these regimens with regard to infection elimination in the endometrium and fallopian tubes or determined the incidence of long-term complications (e.g., tubal infertility and ectopic pregnancy) after antimicrobial regimens. The optimal treatment regimen and long-term outcome of early treatment of women with subclinical PID are unknown. All regimens used to treat PID should also be effective against N. gonorrhoeae and C. trachomatis because negative endocervical screening for these organisms does not rule out upper genital tract infection. Anaerobic bacteria have been isolated from the upper genital tract of women who have PID, and data from in vitro studies have revealed that some anaerobes (e.g., Bacteroides fragilis) can cause tubal and epithelial destruction. BV is often present among women who have PID. Addition of metronidazole to IM or oral PID regimens more effectively eradicates anaerobic organisms from the upper genital tract. Until treatment regimens that do not cover anaerobic microbes have been demonstrated to prevent long-term sequelae (e.g., infertility and ectopic pregnancy) as successfully as the regimens that are effective against these microbes, using regimens with anaerobic activity should be considered. Treatment should be initiated as soon as the presumptive diagnosis has been made because prevention of long-term sequelae is dependent on early administration of recommended antimicrobials. For women with PID of mild or moderate clinical severity, parenteral and oral regimens appear to have similar efficacy. The decision of whether hospitalization is necessary should be based on provider judgment and whether the woman meets any of the following criteria:
- Surgical emergencies (e.g., appendicitis) cannot be excluded
- Tubo-ovarian abscess
- Pregnancy
- Severe illness, nausea and vomiting, or oral temperature >38.5°C (101°F)
- Unable to follow or tolerate an outpatient oral regimen
- No clinical response to oral antimicrobial therapy
No evidence is available to indicate that adolescents have improved outcomes from hospitalization for treatment of PID, and the clinical response to outpatient treatment is similar among younger and older women. The decision to hospitalize adolescents with acute PID should be based on the same criteria used for older women.
Parenteral Treatment
Randomized trials have demonstrated the efficacy of parenteral regimens. Clinical experience should guide decisions regarding transition to oral therapy, which usually can be initiated within 24–48 hours of clinical improvement. For women with tubo-ovarian abscesses, >24 hours of inpatient observation is recommended.
Ceftriaxone 1 g IV every 24 hours
PLUS
Doxycycline 100 mg orally or IV every 12 hours
PLUS
Metronidazole 500 mg orally or IV every 12 hours
OR
Cefotetan 2 g IV every 12 hours
PLUS
Doxycycline 100 mg orally or IV every 12 hours
OR
Cefoxitin 2 g IV every 6 hours
PLUS
Doxycycline 100 mg orally or IV every 12 hours
Because of the pain associated with IV infusion, doxycycline should be administered orally when possible. Oral and IV administration of doxycycline and metronidazole provide similar bioavailability. Oral metronidazole is well absorbed and can be considered instead of IV for women without severe illness or tubo-ovarian abscess when possible. After clinical improvement with parenteral therapy, transition to oral therapy with doxycycline 100 mg 2 times/day and metronidazole 500 mg 2 times/day is recommended to complete 14 days of antimicrobial therapy.
Alternative Parenteral Regimens
Only limited data are available to support using other parenteral second- or third- generation cephalosporins (e.g., ceftizoxime or cefotaxime). Because these cephalosporins are less active than cefotetan or cefoxitin against anaerobic bacteria, the addition of metronidazole should be considered.
Ampicillin-sulbactam plus doxycycline has been investigated in at least one clinical trial and has broad-spectrum coverage. Ampicillin-sulbactam plus doxycycline is effective against C. trachomatis, N. gonorrhoeae, and anaerobes for women with tubo-ovarian abscess. Another trial demonstrated short-term clinical cure rates with azithromycin monotherapy or combined with metronidazole.
When using the clindamycin and gentamicin alternative parenteral regimen, women with clinical improvement after 24–48 hours can be transitioned to clindamycin (450 mg orally 4 times/day) or doxycycline (100 mg orally 2 times/day) to complete the 14-day therapy. However, when tubo-ovarian abscess is present, clindamycin (450 mg orally 4 times/day) or metronidazole (500 mg orally 2 times/day) should be used to complete 14 days of therapy with oral doxycycline to provide more effective anaerobic coverage.
Ampicillin-sulbactam 3 g IV every 6 hours
PLUS
Doxycycline 100 mg orally or IV every 12 hours
OR
Clindamycin 900 mg IV every 8 hours
PLUS
Gentamicin loading dose IV or IM (2 mg/kg body weight), followed by a maintenance dose (1.5 mg/kg body weight) every 8 hours; single daily dosing (3–5 mg/kg body weight) can be substituted
Intramuscular or Oral Treatment
IM or oral therapy can be considered for women with mild-to-moderate acute PID because the clinical outcomes among women treated with these regimens are similar to those treated with IV therapy (1158). Women who do not respond to IM or oral therapy within 72 hours should be reevaluated to confirm the diagnosis and be administered therapy IV.
Ceftriaxone 500 mg IM in a single dose*
PLUS
Doxycycline 100 mg orally 2 times/day for 14 days
WITH
Metronidazole 500 mg orally 2 times/day for 14 days
OR
Cefoxitin 2 g IM in a single dose and Probenecid 1 g orally administered concurrently in a single dose
PLUS
Doxycycline 100 mg orally 2 times/day for 14 days
WITH
Metronidazole 500 mg orally 2 times/day for 14 days
OR
Other parenteral third-generation cephalosporin (e.g., ceftizoxime or cefotaxime)
PLUS
Doxycycline 100 mg orally 2 times/day for 14 days
WITH
Metronidazole 500 mg orally 2 times/day for 14 days
*For persons weighing >150 kg (~300 lbs.) with documented gonococcal infection, 1 g of ceftriaxone should be administered.
These regimens provide coverage against frequent etiologic agents of PID; however, the optimal choice of a cephalosporin is unclear. Cefoxitin, a second-generation cephalosporin, has better anaerobic coverage than ceftriaxone, and, in combination with probenecid and doxycycline, has been effective in short-term clinical response among women with PID. Ceftriaxone has better coverage against N. gonorrhoeae. The addition of metronidazole to these regimens provides extended coverage against anaerobic organisms and will also effectively treat BV, which is frequently associated with PID.
Alternative Intramuscular or Oral Regimens
No data have been published regarding use of oral cephalosporins for treating PID. As a result of the emergence of quinolone-resistant N. gonorrhoeae, regimens that include a quinolone agent are not recommended for PID treatment. However, if the patient has cephalosporin allergy, the community prevalence and individual risk for gonorrhea are low, and follow-up is likely, alternative therapy can be considered with one of the following alternative regimens: 1) levofloxacin 500 mg orally once daily in combination with metronidazole 500 mg orally 2 times/day for 14 days, 2) moxifloxacin 400 mg orally once daily for 14 days, or 3) azithromycin 500 mg IV daily for 1–2 doses, followed by 250 mg orally daily for a total azithromycin duration of 7 days or in combination with metronidazole 500 mg 3 times/day for 12–14 days (1178-1181). Moxifloxacin is the preferred quinolone antimicrobial for M. genitalium infections; however, the importance of providing coverage for M. genitalium is unknown. Diagnostic tests for gonorrhea should be obtained before starting therapy, and persons should be managed as follows:
- If a culture for gonorrhea is positive, treatment should be based on results of antimicrobial susceptibility testing.
- If the isolate is determined to be quinolone-resistant N. gonorrhoeae or if antimicrobial susceptibility cannot be assessed (e.g., if only NAAT testing is available), consultation with an infectious disease specialist is recommended.
Other Management Considerations
To minimize disease transmission, women should be instructed to abstain from sexual intercourse until therapy is complete, symptoms have resolved, and sex partners have been treated (see Chlamydial Infections; Gonococcal Infections). All women who receive a diagnosis of PID should be tested for gonorrhea, chlamydia, HIV, and syphilis. The value of testing women with PID for M. genitalium is unknown (see Mycoplasma genitalium). All contraceptive methods can be continued during treatment.
Follow-Up
Women should demonstrate clinical improvement (e.g., defervescence; reduction in direct or rebound abdominal tenderness; and reduction in uterine, adnexal, and cervical motion tenderness) <3 days after therapy initiation. If no clinical improvement has occurred <72 hours after outpatient IM or oral therapy, then hospitalization, assessment of the antimicrobial regimen, and additional diagnostics, including consideration of diagnostic laparoscopy for alternative diagnoses, are recommended. All women who have received a diagnosis of chlamydial or gonococcal PID should be retested 3 months after treatment, regardless of whether their sex partners have been treated (753). If retesting at 3 months is not possible, these women should be retested whenever they next seek medical care <12 months after treatment.
Management of Sex Partners
Persons who have had sexual contact with a partner with PID during the 60 days preceding symptom onset should be evaluated, tested, and presumptively treated for chlamydia and gonorrhea, regardless of the PID etiology or pathogens isolated. If the last sexual intercourse was >60 days before symptom onset or diagnosis, the most recent sex partner should be treated. Sex partners of persons who have PID caused by C. trachomatis or N. gonorrhoeae frequently are asymptomatic. Arrangements should be made to link sex partners to care. If linkage is delayed or unlikely, EPT is an alternative approach to treating sex partners who have chlamydial or gonococcal infection (see Partner Services). Partners should be instructed to abstain from sexual intercourse until they and their sex partners have been treated (i.e., until therapy is completed and symptoms have resolved, if originally present).
Special Considerations
Drug Allergy, Intolerance, and Adverse Reactions
The risk for penicillin cross-reactivity is highest with first-generation cephalosporins but is negligible between the majority of second-generation (e.g., cefoxitin) and all third-generation (e.g., ceftriaxone) cephalosporins (see Management of Persons Who Have a History of Penicillin Allergy).
Pregnancy
Pregnant women suspected of having PID are at high risk for maternal morbidity and preterm delivery. These women should be hospitalized and treated with IV antimicrobials in consultation with an infectious disease specialist.
HIV Infection
Differences in PID clinical manifestations among women with HIV infection and those without have not been well delineated (1182). In early observational studies, women with HIV infection and PID were more likely to require surgical intervention. More comprehensive observational and controlled studies have demonstrated that women with HIV infection and PID have similar symptoms, compared with women without HIV, except they are more likely to have a tubo-ovarian abscess. Women with HIV responded equally well to recommended parenteral and IM or oral antibiotic regimens as women without HIV. The microbiologic findings for women with HIV and women without HIV were similar, except women with HIV had higher rates of concomitant M. hominis and streptococcal infections. These data are insufficient for determining whether women with HIV infection and PID require more aggressive management (e.g., hospitalization or IV antimicrobial regimens).
Intrauterine Devices
IUDs are one of the most effective contraceptive methods. Copper-containing and levonorgestrel-releasing IUDs are available in the United States. The risk for PID associated with IUD use is primarily confined to the first 3 weeks after insertion. If an IUD user receives a diagnosis of PID, the IUD does not need to be removed. However, the woman should receive treatment according to these recommendations and should have close clinical follow-up. If no clinical improvement occurs within 48–72 hours of initiating treatment, providers should consider removing the IUD. A systematic review of evidence demonstrated that treatment outcomes did not differ between women with PID who retained the IUD and those who had the IUD removed. These studies primarily included women using copper-containing or other nonhormonal IUDs. No studies are available regarding treatment outcomes among women using levonorgestrel-releasing IUDs.
*CDC 2021 STD Treatment Guidelines
Retrieved 10 November 2023
