This sexually-transmitted disease is caused by a gram negative diplococcus.
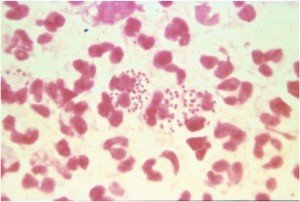
The organism grows easily in the cervical canal, where it can spread to the fallopian tubes, causing PID. It may also infect the urethra, rectum or pharynx.
Many (perhaps most) women harboring the gonococcus will have no symptoms, but others complain of purulent vaginal discharge, pelvic pain, and deep dyspareunia. There may be no significant pelvic findings, but mucopurulent cervical discharge, pain on motion of the cervix, and tenderness in the adnexa are all classical.
The diagnosis is often made on the basis of clinical suspicion but can be confirmed with chocolate agar culture or gram stain.
Dual Therapy for Gonococcal Infections*
On the basis of experience with other microbes that have developed antimicrobial resistance rapidly, a theoretical basis exists for combination therapy using two antimicrobials with different mechanisms of action (e.g., a cephalosporin plus azithromycin) to improve treatment efficacy and potentially slow the emergence and spread of resistance to cephalosporins. Use of azithromycin as the second antimicrobial is preferred to doxycycline because of the convenience and compliance advantages of single-dose therapy and the substantially higher prevalence of gonococcal resistance to tetracycline than to azithromycin among GISP isolates, particularly in strains with elevated cefixime MICs. In addition, clinical trials have demonstrated the efficacy of azithromycin 1 g for the treatment of uncomplicated urogenital GC.
Limited data suggest that dual treatment with azithromycin might enhance treatment efficacy for pharyngeal infection when using oral cephalosporins. In addition, persons infected with N. gonorrhoeae frequently are coinfected with C. trachomatis; this finding has led to the longstanding recommendation that persons treated for gonococcal infection also be treated with a regimen that is effective against uncomplicated genital C. trachomatis infection, further supporting the use of dual therapy that includes azithromycin.
Uncomplicated Gonococcal Infections of the Cervix, Urethra, and Rectum
Recommended Regimen
- Ceftriaxone 250 mg IM in a single dose
PLUS - Azithromycin 1g orally in a single dose
As dual therapy, ceftriaxone and azithromycin should be administered together on the same day, preferably simultaneously and under direct observation. Ceftriaxone in a single injection of 250 mg provides sustained, high bactericidal levels in the blood. Extensive clinical experience indicates that ceftriaxone is safe and effective for the treatment of uncomplicated gonorrhea at all anatomic sites, curing 99.2% of uncomplicated urogenital and anorectal and 98.9% of pharyngeal infections in clinical trials. No clinical data exist to support use of doses of ceftriaxone >250 mg.
Single-dose injectable cephalosporin regimens (other than ceftriaxone 250 mg IM) that are safe and generally effective against uncomplicated urogenital and anorectal gonococcal infections include ceftizoxime (500 mg IM), cefoxitin (2 g IM with probenecid 1 g orally), and cefotaxime (500 mg IM). None of these injectable cephalosporins offer any advantage over ceftriaxone for urogenital infection, and efficacy for pharyngeal infection is less certain. Several other antimicrobials are active against N. gonorrhoeae, but none have substantial advantages over the recommended regimen, and efficacy data (especially for pharyngeal infection) are limited.
Alternative Regimens
If ceftriaxone is not available:
- Cefixime 400 mg orally in a single dose
PLUS - Azithromycin 1 g orally in a single dose
A 400-mg oral dose of cefixime should only be considered as an alternative cephalosporin regimen because it does not provide as high, nor as sustained, bactericidal blood levels as a 250-mg dose of ceftriaxone; further, it demonstrates limited efficacy for treatment of pharyngeal gonorrhea (92.3% cure; 95% confidence interval [CI] = 74.9%–99.1%); in older clinical studies, cefixime cured 97.5% of uncomplicated urogenital and anorectal gonococcal infections (95% CI = 95.4%–99.8%).
The increase in the prevalence of isolates obtained through GISP with elevated cefixime MICs might indicate early stages of development of clinically significant gonococcal resistance to cephalosporins. CDC anticipates that rising cefixime MICs soon will result in declining effectiveness of cefixime for the treatment of urogenital gonorrhea. Furthermore, as cefixime becomes less effective, continued used of cefixime might hasten the development of resistance to ceftriaxone, a safe, well-tolerated, injectable cephalosporin and the last antimicrobial known to be highly effective in a single dose for treatment of gonorrhea at all anatomic sites of infection. Other oral cephalosporins (e.g., cefpodoxime and cefuroxime) are not recommended because of inferior efficacy and less favorable pharmacodynamics.
Because of the prevalence of tetracycline resistance among GISP isolates, particularly those with elevated cefixime MICs, the use of azithromycin as the second antimicrobial is preferred. However, in the case of azithromycin allergy, doxycycline (100 mg orally twice a day for 7 days) can be used in place of azithromycin as an alternative second antimicrobial when used in combination with ceftriaxone or cefixime.
In a recent clinical trial, dual treatment of uncomplicated, urogenital gonorrhea with single doses of oral gemifloxacin 320 mg plus oral azithromycin 2 g was associated with cure rates of 99.5% (lower one-sided 95% CI bound = 97.6%), and dual treatment with single doses of intramuscular gentamicin 240 mg plus oral azithromycin 2 g cured 100% of cases (lower one-sided 95% CI bound = 98.5%). This trial was not powered to provide reliable estimates of the efficacy of these regimens for treatment of rectal or pharyngeal infection, but both regimens cured the few extragenital infections among study participants. Either of these regimens might be considered as alternative treatment options in the presence of cephalosporin allergy. However, gastrointestinal adverse events might limit their use: 7.7% of patients treated with gemifloxacin plus azithromycin and 3.3% of patients treated with gentamicin plus azithromycin vomited within 1 hour of medication administration, necessitating retreatment with a ceftriaxone and azithromycin.
Spectinomycin, which is useful in persons who cannot tolerate cephalosporins, is expensive, has poor efficacy against pharyngeal infection (51.8%; 95% CI = 38.7%–64.9%), and is not being produced in the United States (570). However, it has been effective in clinical trials, curing 98.2% of uncomplicated urogenital and anorectal gonococcal infections. When available, spectinomycin is an effective alternative for the treatment of urogenital and anorectal infection.
Monotherapy with azithromycin 2 g orally as a single dose has been demonstrated to be 99.2% effective against uncomplicated urogenital gonorrhea (95% CI = 97.3%–99.9%). However, monotherapy is no longer recommended because of concerns over the ease with which N. gonorrhoeae can develop resistance to macrolides, and because several studies have documented azithromycin treatment failures. Strains of N. gonorrhoeaecirculating in the United States are not adequately susceptible to penicillins, tetracyclines, and older macrolides (e.g., erythromycin), and thus use of these antimicrobials cannot be recommended.
Uncomplicated Gonococcal Infections of the Pharynx
Most gonococcal infections of the pharynx are asymptomatic and can be relatively common in some populations. Gonococcal infections of the pharynx are more difficult to eradicate than are infections at urogenital and anorectal sites. Few antimicrobial regimens, including those involving oral cephalosporins, can reliably cure >90% of gonococcal pharyngeal infections. Providers should ask their patients with urogenital or rectal GC about oral sexual exposure; if reported, patients should be treated with a regimen with acceptable efficacy against pharyngeal gonorrhea infection.
Recommended Regimen
- Ceftriaxone 250 mg IM in a single dose
PLUS - Azithromycin 1 g orally in a single dose
Other Management Considerations
To maximize adherence with recommended therapies and reduce complications and transmission, medication for gonococcal infection should be provided on site and directly observed. If medications are not available when treatment is indicated, linkage to an STD treatment facility should be provided for same-day treatment. To minimize disease transmission, persons treated for gonorrhea should be instructed to abstain from sexual activity for 7 days after treatment and until all sex partners are adequately treated (7 days after receiving treatment and resolution of symptoms, if present). All persons who receive a diagnosis of gonorrhea should be tested for other STDs, including chlamydia, syphilis, and HIV.
Follow-Up
A test-of-cure is not needed for persons who receive a diagnosis of uncomplicated urogenital or rectal gonorrhea who are treated with any of the recommended or alternative regimens; however, any person with pharyngeal gonorrhea who is treated with an alternative regimen should return 14 days after treatment for a test-of cure using either culture or NAAT. If the NAAT is positive, effort should be made to perform a confirmatory culture before retreatment. All positive cultures for test-of-cure should undergo antimicrobial susceptibility testing.
Symptoms that persist after treatment should be evaluated by culture for N. gonorrhoeae (with or without simultaneous NAAT), and any gonococci isolated should be tested for antimicrobial susceptibility. Persistent urethritis, cervicitis, or proctitis also might be caused by other organisms (see Urethritis, Cervicitis, and Proctitis sections).
A high prevalence of N. gonorrhoeae infection has been observed among men and women previously treated for gonorrhea (86,480,481,577). Rather than signaling treatment failure, most of these infections result from reinfection caused by failure of sex partners to receive treatment or the initiation of sexual activity with a new infected partner, indicating a need for improved patient education and treatment of sex partners. Men or women who have been treated for gonorrhea should be retested 3 months after treatment regardless of whether they believe their sex partners were treated. If retesting at 3 months is not possible, clinicians should retest whenever persons next present for medical care within 12 months following initial treatment.
Management of Sex Partners
Recent sex partners (i.e., persons having sexual contact with the infected patient within the 60 days preceding onset of symptoms or gonorrhea diagnosis) should be referred for evaluation, testing, and presumptive dual treatment. If the patient’s last potential sexual exposure was >60 days before onset of symptoms or diagnosis, the most recent sex partner should be treated. To avoid reinfection, sex partners should be instructed to abstain from unprotected sexual intercourse for 7 days after they and their sexual partner(s) have completed treatment and after resolution of symptoms, if present.
For heterosexual men and women with gonorrhea for whom health department partner-management strategies are impractical or unavailable and whose providers are concerned about partners’ access to prompt clinical evaluation and treatment, EPT with cefixime 400 mg and azithromycin 1 g can be delivered to the partner by the patient, a disease investigation specialist, or a collaborating pharmacy as permitted by law (see Partner Services).
With this approach, provision of medication must be accompanied by written materials to educate partners about their exposure to gonorrhea, the importance of therapy, and when to seek clinical evaluation for adverse reactions or complications. Educational materials for female partners should include information about the importance of seeking medical evaluation for PID (especially if symptomatic); undertreatment of PID in female partners and missed opportunities to diagnose other STDs in women are of concern. EPT should not be considered a routine partner management strategy in MSM with gonorrhea because of a high risk for coexisting infections (especially HIV infection) and because no data exist on efficacy in this population.
Special Considerations
Allergy, Intolerance, and Adverse Reactions
Allergic reactions to first-generation cephalosporins occur in <2.5% of persons with a history of penicillin allergy and are uncommon with third-generation cephalosporins (e.g., ceftriaxone and cefixime). Use of ceftriaxone or cefixime is contraindicated in persons with a history of an IgE-mediated penicillin allergy (e.g., anaphylaxis, Stevens Johnson syndrome, and toxic epidermal necrolysis). Data are limited regarding alternative regimens for treating gonorrhea among persons who have either a cephalosporin or IgE-mediated penicillin allergy. Potential therapeutic options are dual treatment with single doses of oral gemifloxacin 320 mg plus oral azithromycin 2 g or dual treatment with single doses of intramuscular gentamicin 240 mg plus oral azithromycin 2 g. Spectinomycin for treatment of urogenital and anorectal gonorrhea can be considered when available. Providers treating persons with cephalosporin or IgE-mediated penicillin allergy should consult an infectious-disease specialist.
Pregnancy
Pregnant women infected with N. gonorrhoeae should be treated with dual therapy consisting of ceftriaxone 250 mg in a single IM dose and azithromycin 1 g orally as a single dose. When cephalosporin allergy or other considerations preclude treatment with this regimen and spectinomycin is not available, consultation with an infectious-disease specialist is recommended.
*CDC 2015 STD Treatment Guidelines
