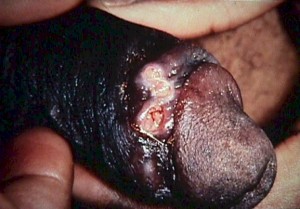
This sexually-transmitted illness begins as a tender, reddened papule filled with pus.
It then breaks down, ulcerates and reveals a grayish, necrotic base with jagged, irregular margins.
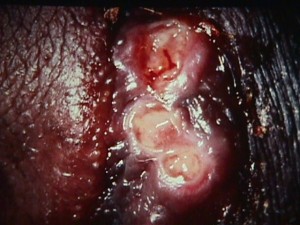
There is no significant induration around the base, unlike primary syphilis. In untreated cases, the lesions may spread and substantial tissue damage may result. Tender, enlarged inguinal lymph nodes are found in 50% of patients.
Hemophilus ducreyi, the causative organism, is difficult to culture, so the diagnosis is made on the basis of history, physical exam and exclusion of other ulcerative diseases of the vulva. A gram-stain from the base of a clean ulcer or aspirate from a bubo may reveal a gram-negative coccobacillus clustered in groups around polymorphonucleocytes (“school of fish ” appearance).
Treatment*
Successful treatment for chancroid cures the infection, resolves the clinical symptoms, and prevents transmission to others. In advanced cases, scarring can result despite successful therapy.
Recommended Regimens
- Azithromycin 1 g orally in a single dose
OR - Ceftriaxone 250 mg IM in a single dose
OR - Ciprofloxacin 500 mg orally twice a day for 3 days
OR - Erythromycin base 500 mg orally three times a day for 7 days
Azithromycin and ceftriaxone offer the advantage of single-dose therapy. Worldwide, several isolates with intermediate resistance to either ciprofloxacin or erythromycin have been reported. However, because cultures are not routinely performed, data are limited regarding the current prevalence of antimicrobial resistance.
Other Management Considerations
Men who are uncircumcised and patients with HIV infection do not respond as well to treatment as persons who are circumcised or HIV-negative. Patients should be tested for HIV infection at the time chancroid is diagnosed. If the initial test results were negative, a serologic test for syphilis and HIV infection should be performed 3 months after the diagnosis of chancroid.
Follow-Up
Patients should be re-examined 3–7 days after initiation of therapy. If treatment is successful, ulcers usually improve symptomatically within 3 days and objectively within 7 days after therapy. If no clinical improvement is evident, the clinician must consider whether 1) the diagnosis is correct, 2) the patient is coinfected with another STD, 3) the patient is infected with HIV, 4) the treatment was not used as instructed, or 5) the H. ducreyi strain causing the infection is resistant to the prescribed antimicrobial. The time required for complete healing depends on the size of the ulcer; large ulcers might require >2 weeks. In addition, healing is slower for some uncircumcised men who have ulcers under the foreskin. Clinical resolution of fluctuant lymphadenopathy is slower than that of ulcers and might require needle aspiration or incision and drainage, despite otherwise successful therapy. Although needle aspiration of buboes is a simpler procedure, incision and drainage might be preferred because of reduced need for subsequent drainage procedures.
Management of Sex Partners
Regardless of whether symptoms of the disease are present, sex partners of patients who have chancroid should be examined and treated if they had sexual contact with the patient during the 10 days preceding the patient’s onset of symptoms.
Special Considerations
Pregnancy
Data suggest ciprofloxacin presents a low risk to the fetus during pregnancy, with a potential for toxicity during breastfeeding. Alternate drugs should be used during pregnancy and lactation. No adverse effects of chancroid on pregnancy outcome have been reported.
HIV Infection
Persons with HIV infection who have chancroid should be monitored closely because they are more likely to experience treatment failure and to have ulcers that heal slowly. Persons with HIV infection might require repeated or longer courses of therapy, and treatment failures can occur with any regimen. Data are limited concerning the therapeutic efficacy of the recommended single-dose azithromycin and ceftriaxone regimens in persons with HIV infection.
* The material from “Treatment” to the end of this page was drawn from the 2015 Sexually Transmitted Diseases Treatment Guidelines, CDC
