Yeast Infections
Vaginal yeast infections are common, monilial overgrowths in the vagina
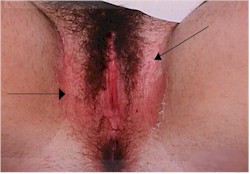
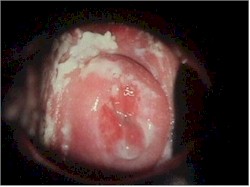
and vulvar areas, characterized by itching,dryness, and a thick, cottage-cheese appearing
vaginal discharge. The vulva may be reddened and irritated to the point of tenderness.
These infections are particularly troublesome in operational settings where they are
both frequent and annoying. Yeast thrives in damp, hot environments and women in such
circumstances are predisposed toward these infections.Women who take broad-spectrum
antibiotics are also predisposed towards these infections because of loss of the normal
vaginal bacterial flora.
Yeast organisms are normally present in most vaginas, but in small numbers. A yeast
infection, then, is not merely the presence of yeast, but the concentration of yeast in
such large numbers as to cause the typical symptoms of itching, burning and discharge.
Likewise, a "cure" doesn't mean eradication of all yeast organisms from the
vagina. Even if eradicated, they would soon be back because that is where they normally
live. A cure means that the concentration of yeast has been restored to normal and
symptoms have resolved.
The diagnosis is often made by history alone, and enhanced by the classical appearance
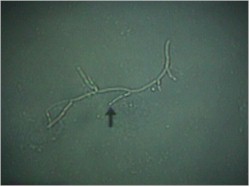
of a dry, cheesy vaginal discharge. It can be confirmed by microscopic visualization of clusters of thread-like,
branching Monilia organisms when the discharge is mixed with KOH.
Treatment consists of an oral antifungal agent,
- Fluconazole 150 mg oral tablet, one tablet in single dose,
or intravaginal agents:
- Butoconazole 2% cream 5 g intravaginally for 3 days
- Clotrimazole 1% cream 5 g intravaginally for 7-14 days
- Clotrimazole 100 mg vaginal tablet for 7 days
- Clotrimazole 100 mg vaginal tablet, two tablets for 3 days
- Clotrimazole 500 mg vaginal tablet, one tablet in a single application
- Miconazole 2% cream 5 g intravaginally for 7 days
- Miconazole 200 mg vaginal suppository, one suppository for 3 days
- Miconazole 100 mg vaginal suppository, one suppository for 7 days
- Nystatin 100,000-unit vaginal tablet, one tablet for 14 days
- Tioconazole 6.5% ointment 5 g intravaginally in a single application
- Terconazole 0.4% cream 5 g intravaginally for 7 days
- Terconazole 0.8% cream 5 g intravaginally for 3 days
- Terconazole 80 mg vaginal suppository, one suppository for 3 days.
If none of these products are available, douching with a weak acid solution (2
teaspoons of vinegar in a quart of warm water) twice a day will help restore an acid pH to
the vagina, inhibiting yeast proliferation. Stop douching when symptoms have resolved as
the douche itself tends to remove some of the protective mucous within the vagina.
Whenever the skin of the vulva is involved, more frequent treatment for a longer period
of time may be necessary.
Reoccurrences are common and can be treated the same as for initial infections. For
chronic recurrences, many patients find the use of a single applicator of
Monistat 7 at
the onset of itching will abort the attack completely. Sexual partners need not be treated
unless they are symptomatic.
CDC Treatment Guidelines
|
These videos are enhancements to the original
Department of the Navy Publication, provided by the Brookside Associates
Medical Education Division |
|
|
|
|
Bureau of Medicine and
Surgery
Department of the Navy
2300 E Street NW
Washington, D.C
20372-5300 |
Operational Obstetrics
& Gynecology - 2nd Edition
The Health Care of Women in Military Settings
CAPT Michael John Hughey, MC, USNR
NAVMEDPUB 6300-2C
January 1, 2000 |
|
|
Approved for public release;
Distribution is unlimited.
The listing of any non-Federal product in this CD is not an endorsement of the
product itself, but simply an acknowledgement of the source.
Bureau of Medicine and Surgery
Department of the Navy
2300 E Street NW
Washington, D.C
20372-5300 |
Operational Medicine
Health Care in Military Settings
CAPT Michael John Hughey, MC, USNR
NAVMED P-5139
January 1, 2001 |
United States Special Operations
Command
7701 Tampa Point Blvd.
MacDill AFB, Florida
33621-5323 |
*This web version is provided by
The Brookside Associates Medical Education Division. It contains
original contents from the official US Navy NAVMED P-5139, but has been
reformatted for web access and includes advertising and links that were not
present in the original version. This web version has not been approved by the
Department of the Navy or the Department of Defense. The presence of any
advertising on these pages does not constitute an endorsement of that product or
service by either the US Department of Defense or the Brookside Associates. The
Brookside Associates is a private organization, not affiliated with the United
States Department of Defense.
Contact Us · Other
Brookside Products

|
|
Operational Medicine 2001
Contents
|
|