Anemia
|
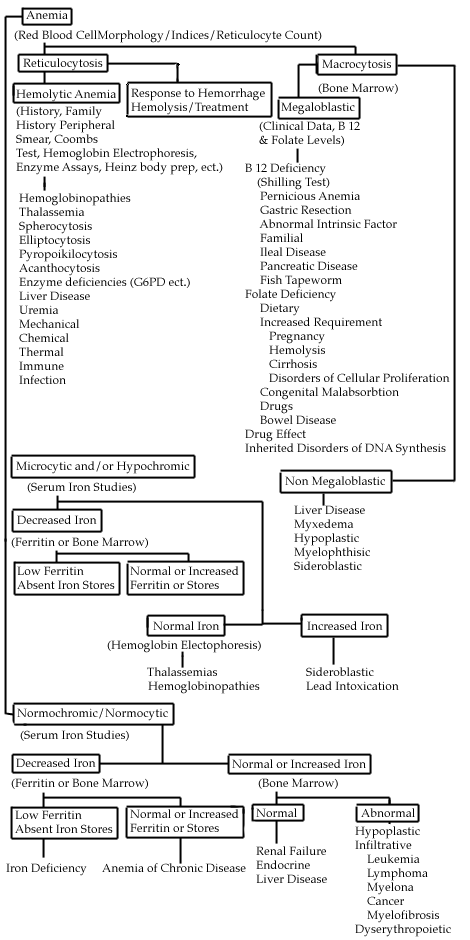
Anemia is defined as a decreased level of hemoglobin more than 2 standard deviations below the expected mean for age and sex. In quickly assessing anemia, the presence or absence of symptoms should be determined. Patients with slowly developing anemias will often be completely compensated and have few if any symptoms. Conversely, patients with rapidly developing anemias (i.e., from bleeding, hemolysis) may present with hypotension, tachycardia, weakness, and fatigue. Sometimes the signs and symptoms will point to the diagnosis (pagophagia-ice craving-and iron deficiency; melena-GI bleeding; glossitis and loss of proprioception in B12 deficiency). The family history (sickle cell disease) and exposure history (drugs, toxins, alcohol) are also important. The physical examination may also reveal significant lymphadenopathy, splenomegaly, or heme (+) stools. After the patient is determined anemic and the history and physical exam is complete, several simple tests can be performed which may quickly point to the general cause of the anemia. These indicators include the following:
By carefully following these recommendations, a correct diagnosis can often be determined and an appropriate treatment can be prescribed. Classification of Anemias Most anemias can be classified into three broad categories. 1. Hypoproliferative Anemias This group results from ineffective production of hemoglobin (cytoplasmic maturation disorders) or ineffective development of nuclear DNA (nuclear maturation disorders). Reviewing the RBC indices often makes diagnosis. Cytoplasmic maturation defect anemias present with microcytosis (usually <70-75 ul). The major causes of anemias in this group are iron deficiency, thalassemia, lead intoxication, B6 deficiency, and inflammatory anemias/anemia of chronic disease (often but not always with a high ESR). There are 2 major causes of nuclear maturation defects:
These patients usually have an MCV > 110 ul. Generally speaking, if your patient has significant anemia, without any symptoms, (suggesting an anemia that has developed very slowly) and has either very small cells or very large cells, the maturation disorders are likely to be a factor. B12, iron, and folate levels can be easily measured. If low, treatment is generally straightforward. Hemolytic Anemias In the shipboard or field environment, quickly identify anemias that require rapid transfer to a higher echelon of care, such as those due to blood loss (usually GI bleeding) and due to serious bone marrow disease (i.e., acute leukemia). As always in clinical medicine, a careful history and physical exam plus a review of the blood smear will accomplish this goal the majority of the time without requiring specialized testing. Consultation via telephone, email, or VTC is encouraged if any questions arise. Use the following table as a guide for classifying anemias.
Contributing Authors include CAPT George Luiken, MC, USN (1990), and CAPT John C. Phares, MC, USN (1993). Reviewed by CAPT Fred Millard, MC, USN, Department of Hematology/Oncology, Naval Medical Center San Diego, San Diego, CA (1999).
|
Preface · Administrative Section · Clinical Section
The
General Medical Officer Manual , NAVMEDPUB 5134, January 1, 2000
Bureau
of Medicine and Surgery, Department of the Navy, 2300 E Street NW, Washington, D.C.,
20372-5300
This web version of The General Medical Officer Manual, NAVMEDPUB 5134 is provided by The Brookside Associates Medical Education Division. It contains original contents from the official US Navy version, but has been reformatted for web access and includes advertising and links that were not present in the original version. This web version has not been approved by the Department of the Navy or the Department of Defense. The presence of any advertising on these pages does not constitute an endorsement of that product or service by either the Department of Defense or the Brookside Associates. The Brookside Associates is a private organization, not affiliated with the United States Department of Defense. All material in this version is unclassified. This formatting © 2006 Medical Education Division, Brookside Associates, Ltd. All rights reserved.
Home · Textbooks and Manuals · Videos · Lectures · Distance Learning · Training · Operational Safety · Search
This website is dedicated to the development and dissemination of medical information that may be useful to those who practice Operational Medicine. This website is privately-held and not connected to any governmental agency. The views expressed here are those of the authors, and unless otherwise noted, do not necessarily reflect the views of
the Brookside Associates, Ltd., any governmental or private organizations. All writings, discussions, and publications on this website are unclassified.
© 2006 Medical Education Division, Brookside Associates, Ltd. All rights reserved
Other Brookside Products