|
Medical Education Division |
Operational Medicine 2001
Home · Military Medicine · Sick Call · Basic Exams · Medical Procedures · Lab and X-ray · The Pharmacy · The Library · Equipment · Patient Transport · Medical Force Protection · Operational Safety · Operational Settings · Special Operations · Humanitarian Missions · Instructions/Orders · Other Agencies · Video Gallery · Phone Consultation · Forms · Web Links · Acknowledgements · Help · Feedback
Famine-Affected, Refugee, and Displaced Populations: Recommendations for Public Health IssuesMMWR - Vol. 41, No. RR-13Publication date: 07/24/1992Table of Contents
ARTICLE BACKGROUND FAMINE-AFFECTED POPULATIONS REPORTS
RECOMMENDATIONS
POINT OF CONTACT FOR THIS DOCUMENT:
Tables
Figures
ARTICLEU.S. Department of Health and Human Services The MMWR series of publications is published by the Epidemiology Program Office, Centers for Disease Control, Public Health Service, U.S. Department of Health and Human Services, Atlanta, Georgia 30333.
SUGGESTED CITATIONCenters for Disease Control. Famine-Affected, refugee, and displaced populations: recommendations for public health issues. MMWR 1992;41(No. RR-13);(inclusive page numbers).
Centers for Disease Control
The material in this report was prepared for publication by: International
Health Program Office
Andrew A. Vernon, M.D., Director Robert J. Baldwin, Director
The production of this report as an MMWR serial publication was coordinated
in:
Scientific Information and Communications Program
Information Resources Management Branch Use of trade names is for identification only and does not imply endorsement by the Public Health Service or the U.S. Department of Health and Human Services. Copies can be purchased from Superintendent of Documents, U.S. Government Printing Office, Washington, D.C. 20402-9325. Telephone (202)783-3238.
AUTHORSThe following CDC staff members served as authors for this publication: Mike
J. Toole, M.D., DTM&H *
Rita M. Malkki, M.P.H.
The experts listed below contributed to the preparation of this publication:
Paul A. Blake, M.D., M.P.H.
Lisa A. Lee, V.M.D., M.P.H.
Eric E. Mast, M.D., M.P.H.
Phillip I. Nieburg, M.D., M.P.H.
Dixie E. Snider, Jr., M.D. M.P.H.
Richard W. Steketee, M.D., M.P.H.
Roland W. Sutter, M.D., M.P.H.&TM Ronald J. Waldman, M.D., M.P.H.
Strengthening of Epidemiological and Statistical Services Unit Health
Situation and Trend Assessment Division
Ray Yip, M.D., M.P.H. National Center for Chronic Disease Prevention and Health Promotion Special contributions were made by:
Molly (Mary Susan) Bardsley
GLOSSARYAFB Acid-Fast Bacilli ALRI Acute Lower Respiratory Infection ARDS Adult Respiratory Distress Syndrome ARIs Acute Respiratory Infections BCG Bacille Calmette-Guerin CFR Case-Fatality Ratio CMR Crude Mortality Rate CNS Central Nervous System CSB Corn-soya Blend CSF Cerebral Spinal Fluid CSM Corn-soya Milk DPT Diphtheria-Pertussis-Tetanus DSM Dry Skim Milk E-Z Edmonston-Zagreb Vaccine EIS Epidemic Intelligence Service EPI Expanded Programs on Immunization G-6-PD Glucose-6-Phosphate Dehydrogenase Hb Hemogolobin HEM High Energy Milk HIS Health Information System HIV Human Immunodeficiency Virus IU International Units Kcal Kilocalories MCH Maternal and Child Health MOH Ministry of Health MSF Medecins Sans Frontieres MUAC Mid-Upper Arm Circumference NGO Nongovernmental Organization OPV Oral Polio Vaccine ORS Oral Rehydration Solution ORT Oral Rehydration Therapy PAHO Pan American Health Organization PEM Protein Energy Malnutrition PHC Primary Health Care PVO Private Voluntary Organization SFP Supplementary Feeding Program SMX Sulfamethoxazole SP Sulfadoxine-Pyrimethamine STD Sexually Transmitted Disease TB Tuberculosis TFP Therapeutic Feeding Program TMP Trimethoprim TT Tetanus Toxoid UNHCR United Nations High Commissioner for Refugees
UNICEF United Nations International Children's Emergency Fund
USAID United States Agency for International Development
WFH Weight-For-Height WHO World Health Organization WSB Wheat-soya Blend
PREFACEPreparing for the health problems experienced by large populations displaced by natural or man-made disasters is among the greatest challenges facing public health officials in the world today. The diversity of problems experienced in long- and short-term refugee situations demands a diversity of approaches in disease surveillance, control, and prevention. The Centers for Disease Control's experience over the past decade has allowed us to evolve approaches which allow for timely and accurate surveillance data to be generated even in extremely adverse conditions. The resulting prevention activities are well focused on the most important public health problems. These reports and guidelines have been developed by a number of CDC professionals working with international organizations and public health agencies, such as, the Pan American Health Organization, the United Nations High Commissioner for Refugees, the United States Agency for International Development, and the private voluntary organization's of refugee situations. These reports and guidelines reflect our belief that appropriate, cost-effective disease prevention technology can be rapidly applied in most situations that will impact positively the lives of the affected populations. The recommendations underscore our organizational interest and commitment to a global health agenda that will improve the health status of people worldwide. International disaster preparedness and refugee activities are collaborative efforts. CDC efforts are performed jointly with many other governmental, nongovernmental, and international organizations. It is my hope that public health professionals involved in dealing with these issues will find this information useful in their planning, training, and emergency preparedness efforts.
William L. Roper, M.D., M.P.H., Director
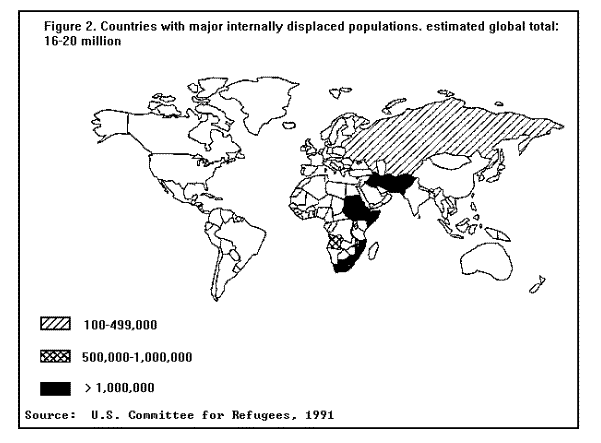
FOREWORDIn the past decade, public health emergencies have occured with great frequency -- and the number of people affected has captured the attention of the world. Many of these emergencies involved some degree of forced population migration, and almost all have been associated with severe food shortages. Natural disasters, such as droughts and floods, have been partially responsible, but the most common causes of these emergencies have been war and civil strife. Since 1984, the number of refugees dependent for their survival on international assistance has more than doubled to a current estimate of approximately 17 million persons -- almost all in developing countries. Kurdish refugees fleeing Iraq captured the world's attention briefly in early 1991, but the desperate plight of many others -- especially the 5 million refugees in Africa -- receives scant attention from the world media. Even more obscure are the estimated 16-20 million displaced persons who are trapped within their countries by civil wars and are unable to cross borders to seek help from the international community. This situation represents an unprecedented challenge to the international public health community. CDC has had a long-standing institutional commitment to the problem of famine-affected, refugee, and displaced populations for many years. During the Nigerian Civil War in the 1960s, 20 Epidemic Intelligence Service officers helped maintain public health programs for millions of displaced civilians, who were deprived of their basic needs by that war. Since then, CDC has provided technical assistance to relief agencies working in most of the world's major refugee emergency communities including those in, for example, Ethiopia, Kenya, Malawi, Pakistan, Somalia, Sudan, Thailand, Turkey, and West Africa. CDC, United Nations agencies, countries of asylum, and private voluntary organizations (PVOs) have attempted to adapt traditional epidemiologic techniques and public health programs to the realities of refugee camps and scattered, famine-affected communities. As a result, a considerable body of knowledge and experience has accumulated and has been documented in various issues of the MMWR. This report represents a compilation of this knowledge for dissemination and for providing guidance on certain technical subjects for those involved in future relief programs. By necessity, this document is unable to cover all aspects of emergency relief. The recommendations provided here will not be effective unless they are supported by adequate preparedness planning, coordination, communications, logistics, personnel management, and relief worker training. Even more critical is ensuring access by relief workers to internally displaced populations -- many needy communities are caught in areas of contested sovereignty. Unless the international community can devise ways of providing assistance to communities in these circumstances, it will be impossible to implement these basic public health programs. Finally, the situation of refugees and displaced persons is a timely reminder of the clear interface between public health and social justice. The most effective measure to prevent the high mortality experienced by these populations would be to eliminate the causes of the violence and conflict from which they fled.
Joe H. Davis, M.D. Famine-Affected, Refugee, and Displaced Populations: Recommendations for Public Health Issues
INTRODUCTIONDuring the past three decades, the most common emergencies affecting the health of large populations in developing countries have involved famine and forced migrations. The public health consequences of mass population displacement have been extensively documented. On some occasions, these migrations have resulted in extremely high rates of mortality, morbidity, and malnutrition. The most severe consequences of population displacement have occurred during the acute emergency phase, when relief efforts are in the early stage. During this phase, deaths -- in some cases -- were 60 times the crude mortality rate (CMR) among non-refugee populations in the country of origin (1). Although the quality of international disaster response efforts has steadily improved, the human cost of forced migration remains high. Since the early 1960s, most emergencies involving refugees and displaced persons have taken place in less developed countries where local resources have been insufficient for providing prompt and adequate assistance. The international community's response to the health needs of these populations has been at times inappropriate, relying on teams of foreign medical personnel with little or no training. Hospitals, clinics, and feeding centers have been set up without assessment of preliminary needs, and essential prevention programs have been neglected. More recent relief programs, however, emphasize a primary health care (PHC) approach, focusing on preventive programs such as immunization and oral rehydration therapy (ORT), promoting involvement by the refugee community in the provision of health services, and stressing more effective coordination and information gathering. The PHC approach offers long-term advantages, not only for the directly affected population, but also for the country hosting the refugees. A PHC strategy is sustainable and strengthens the national health development program.
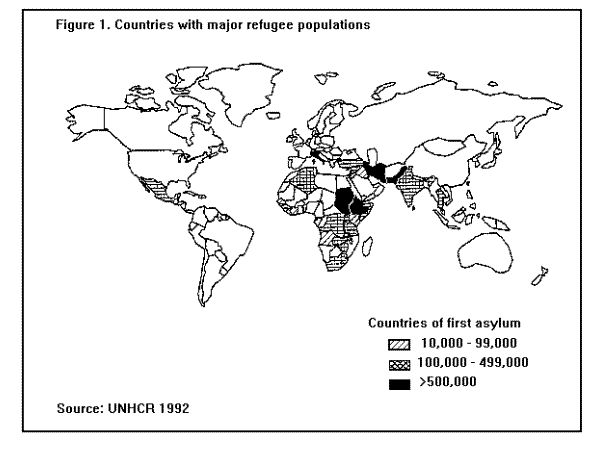
BACKGROUNDClassification of DisastersOne way of describing the evolution of disasters is in terms of a "trigger event" leading to "primary effects" and "secondary effects" on vulnerable groups in the population (2). In the case of a rapid-onset natural disaster like an earthquake, the primary effects, deaths and injuries, may be high, but there are few secondary effects. In the case of slow-onset natural disasters like drought and manmade disasters, like war and civil strife, the secondary effects (i.e., decreased food availability, environmental damage, and population displacement) may lead to a higher delayed death toll than that of the initial event. Although population displacement may result from a number of different types of disasters -- manmade and natural -- the two most common recent trigger events have been food deficits and war. In many parts of the world where food shortages have become common, war and civil strife are major causative factors. Consequently, war, food deficits, famine, and population displacement have been inextricably linked risk factors for increased mortality in certain large populations in Africa, Asia, Latin America, and the Middle East. (Figure 1) Countries with major refugee populations (Table 1) Refugees and asylumseekers by geographic region, July 1991 (Figure 2) Countries with major displaced populations, estimated global total: 16- 20 million The purpose of this report is to describe the public health consequences of famine and population displacement in developing countries and to present the most current recommendations on public health programs of major importance. Refugee and Displaced Populations The 1951 United Nations Convention defines a refugee as "Any person who owing to a well founded fear of being persecuted for reasons of race, religion, nationality, membership of a particular social group or political opinion is outside the country of his nationality and is unable, or owing to fear is unwilling to avail himself of the protection of that country; or who, not having a nationality and being outside the country of his former habitual residence, is unable, or having such fear is unwilling to return to it" (3). In 1969, the Organization of African Unity expanded this definition to include persons fleeing from war, civil disturbance, and violence of any kind (4). These definitions, however, exclude persons who leave their country of origin to seek economic betterment, as well as persons or groups who may flee their homes for the above or other reasons, yet remain within the borders of their own country. There are few, if any, international regulations covering these internally displaced populations, yet it is estimated that more than half of all displaced persons worldwide are living within the borders of their home country (5).
FAMINE-AFFECTED POPULATIONSDefinition and CausesFamine has been defined as "a condition of populations in which a substantial increase in deaths is associated with inadequate food consumption" (6). Famine does not necessarily arise solely from problems of food production. Natural disasters (e.g., drought or crop infestations) may act as triggers, but lack of sufficient food for consumption may be due to economic collapse and loss of purchasing power in some sections of the population, (i.e., the Indian famine of 1972). In early 1992, efforts to assess the impact of sudden economic changes in the republics of the former Soviet Union have focused on income and food price indicators. In Russia, elderly pensioners were identified as a vulnerable group among whom the income-to-food cost ratio was estimated to be 1:2 in mid-January (7). Other causes of famine have included disruption of food production and marketing by armed conflict (i.e., Biafra in 1968, Sudan in 1988, and Somalia in 1991) and widespread civil disturbances (i.e., Zaire in 1991). Famine is usually caused by the amplification of a pre-existing condition characterized by widespread poverty, intractable debt, underemployment, and high malnutrition prevalence. Under these conditions, a large percentage of the population may routinely experience starvation. When additional burdens related to the production or availability of food arise, generalized starvation occurs rapidly. In recent years, frequent crop failures in Ethiopia, Somalia, Sudan, and the Sahelian countries of Africa have been attributed to progressive deterioration of the environment, including deforestation, desertification, and poor agricultural practices. Populations experiencing famine may or may not displace themselves in order to improve food availability. Initially, male family members may migrate to cities or neighboring countries to seek employment. During a full-scale famine, whole families and villages may flee to other regions or countries in a desperate search for food. In most of the major population displacements of the past 20 years, however, people have been forced to flee because of fear for their physical security caused by war or civil strife. Famine in the absence of violence has generated few of the world's refugees.
Detection of FamineFamines are often assessed and reported in terms of cases, rates, or degrees of malnutrition, or numbers of deaths from malnutrition. These parameters have been classified as "trailing" indicators and are not useful for early famine detection and the initiation of prevention or mitigation measures. More important in the early detection of famine are "leading" and "intermediate" indicators that reflect changes in the economic, social, and environmental factors that influence the evolution of food shortages and famine. The leading and intermediate indicators will be useful if they trigger early interventions aimed at ensuring adequate food supplies for the population and at maintaining the purchasing power of vulnerable groups. These measures have included temporary government subsidies for food crops, "food-for-work" programs; government-run, fixed-price food shops; rural employment schemes; the distribution of drought-resistant seeds; and the release of food reserves. Effective early warning systems might help avert major population movements, thereby allowing local government and international and private voluntary organizations (PVOs) to provide assistance in situ without major disruption in traditional social structures and lifestyle patterns. Affected communities can be surveyed, needy households identified, food and other relief supplies distributed, and major epidemics averted with greater ease and effectiveness in a stable population than in a temporary refugee settlement. National early warning systems have proved effective in preventing famine during the past decade in India and Botswana (8). When populations are forced to migrate en masse, they usually end up in camps or urban slums characterized by overcrowding, poor sanitation, substandard housing, and limited access to health services. These conditions hamper the effective and equitable distribution of relief supplies and promote the transmission of communicable diseases.
REPORTSThe most direct and obvious results of famine are severe undernutrition and death. While longitudinal studies have demonstrated that undernourished persons -- particularly children -- are at higher risk of mortality, the immediate cause of death is usually a communicable disease. Malnutrition causes an increased case-fatality ratio (CFR) in the most common childhood communicable diseases (i.e., measles, diarrheal disease, malaria, and acute respiratory infections (ARIs)). Those at highest risk of mortality during nonfamine times -- namely, the poor, the elderly, women, and young children -- are the same groups most at risk for the morbidity and mortality caused by famine. In addition, the movement of populations into crowded and unsanitary camps, the violence associated with forced migrations, and the negative psychological effects of fear, uncertainty, and dependency contribute to the health problems experienced by displaced persons.
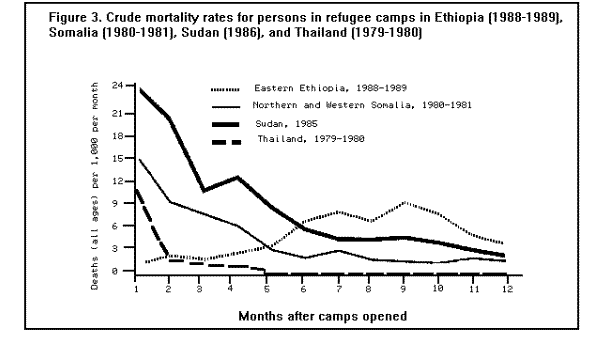
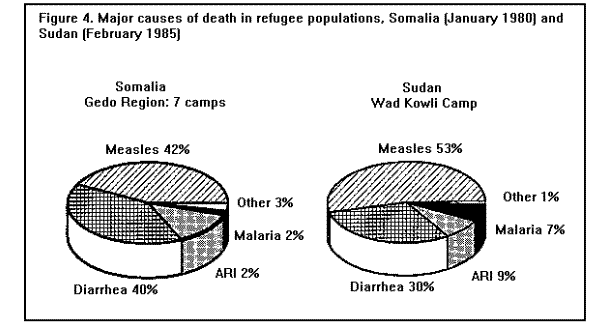
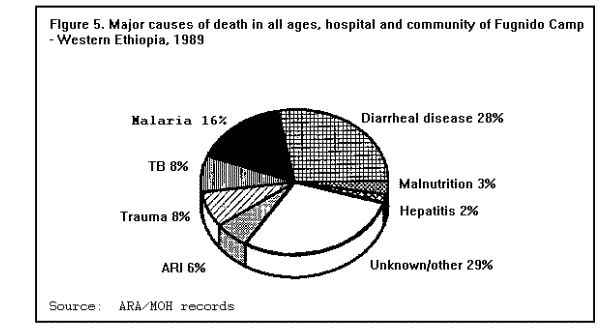
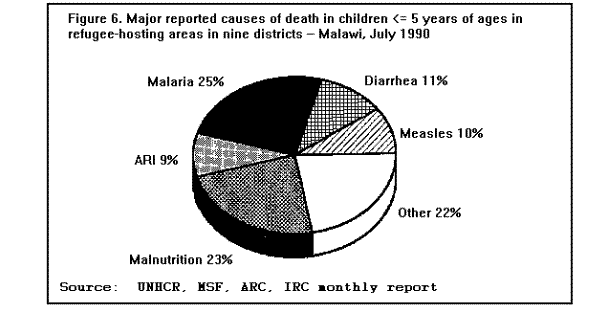
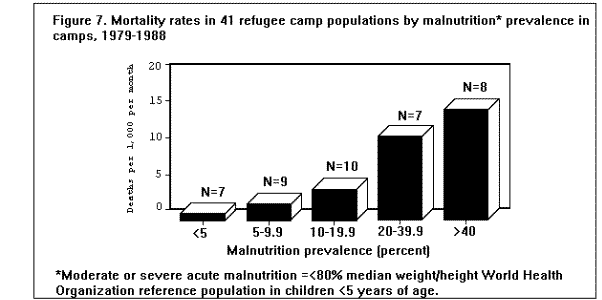
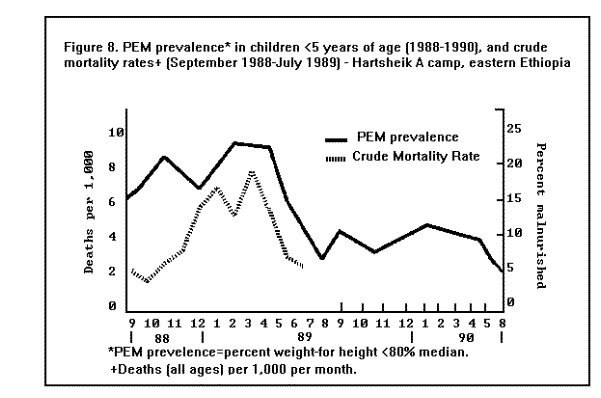
MortalityMortality rates are the most specific indicators of the health status of emergency-affected populations. Mortality rates have been estimated retrospectively from hospital and burial records, or from community-based surveys, and prospectively from 24-hour burial site surveillance. Among the many problems encountered in estimating mortality under emergency conditions are recall bias in surveys, families' failure to report perinatal deaths, inaccurate denominators (overall population size, births, age-specific populations), and lack of standard reporting procedures. In general, bias tends to underestimate mortality rates, since deaths are usually underreported or undercounted, and population size is often exaggerated. Most reports of famine-related mortality have come from populations that have experienced considerable displacement. It is possible that mortality rates are lower in those populations that remain in their original villages and homes. A comparison of mortality in displaced vs. nondisplaced, famine-affected populations is problematic because displacement itself may reflect a more serious baseline situation. Nonetheless, comparisons between displaced and nondisplaced populations during famine on one hand, and between refugees and local, host country populations on the other hand, show that in nearly all cases the displaced and refugee populations experience a markedly higher CMR. The CMRs reported in various refugee, internally displaced, and famine-affected (but nondisplaced) populations, respectively, during the emergency phase of relief operations in the past 15 years are listed in ( Table 2), (Table 3), and (Table 4). These rates are compared with baseline CMRs reported for nonfamine-affected and nondisplaced populations, or, in the case of refugees, with CMRs in their country of origin. CMRs in these tables are expressed as deaths per 1,000 per month to reflect the short reporting periods; comparison rates have been extrapolated from annual CMRs published by the United Nations Children's Fund (UNICEF) (13). Although CMRs reported in refugee emergencies have not been adjusted for age and sex, it is unlikely that demographic differences between refugee and non-refugee populations account for the excess mortality found among many of the latter. Monthly CMRs recorded immediately after the initial influx of Cambodian refugees into Thailand (1979), Ethiopian refugees into Somalia (1980), and Ethiopian refugees into eastern Sudan (1985) were 8.1 to 15.2 times the expected rates. The early death rate among Kurdish refugees in Turkey in April 1991 was 18 times the baseline rates in both Iraq and Turkey. In contrast, among Mozambican refugees in Malawi in 1987, camp-based CMRs were one-third lower than the national CMR reported for Mozambique. A movement of 50,000 refugees from Burundi into Rwanda in 1988 also resulted in minimal mortality once asylum had been attained. The rate of improvement in camp populations has varied considerably. For example, mortality rates decreased rapidly in Cambodian refugee camps in Thailand in 1979-1980 and in the Kurdish camps of Turkey in 1991, but only slow improvement occurred during the initial 8 months in Somalia (1980) and in Sudan (1985). In eastern Ethiopia in 1988-1989, initially low mortality rates among Somali refugees increased after 6 months, reaching a peak at 9 months (Figure 3). Overall, less than 1% of Cambodian refugees in Thai camps died during the first 12 months; 9% of refugees in eastern Sudan died during the same period of time (1). Political and security factors often obstruct the accurate documentation of death rates among internally displaced populations; however, a few situations have been well documented. In Mozambique (1983), Ethiopia (1984-1985), and Sudan (1988), CMRs estimated by surveillance or population-based surveys of internally displaced persons ranged between 4 and 70 times the death rates in nondisplaced populations in the same country. In the Korem area of Ethiopia, CMRs recorded among camp populations displaced by famine in 1985 were 7-10 times those of settled villagers in a similar highland zone affected by the famine. In Monrovia, the capital of Liberia, the death rate among civilians displaced during the 1990 civil war was 7 times the pre-war death rate (Holland MSF, unpublished data, January 1991). As in stable populations in developing countries, age-specific death rates in displaced and refugee populations are highest in children less than 5 years of age. A mortality survey of Kurdish refugees at the Turkey-Iraq border during 1991 revealed that 63% of all deaths occurred among children less than 5 years of age, who comprised approximately 18% of the population (11). Although absolute death rates are highest in infants less than 1 year of age, the relative increase in mortality during emergencies may be highest in children 1-12 years of age (1). Cause-specific mortalityThe major reported causes of death in refugee and displaced populations have been those same diseases that cause high death rates in nondisplaced populations in developing countries: malnutrition, diarrheal diseases, measles, ARIs, and malaria. These diseases consistently account for 60%-95% of all reported causes of death in these populations (Figure 4), ( Figure 5), and (Figure 6). Specific reports on these and other communicable diseases are presented in a later section. In those situations where malnutrition was not classified as an immediate cause of death (i.e., Sudan and Somalia), it was a major underlying factor accounting for the high CFRs from communicable diseases. This synergism between high malnutrition prevalence and increased incidence of communicable diseases explains much of the excess mortality seen in refugee and displaced populations. A study of 42 refugee populations completed in 1989 examined acute protein energy malnutrition (PEM) prevalence and crude unadjusted monthly mortality rates, gathered from 1984-1988. Analysis of the data showed a strong positive association between PEM prevalence and CMRs. Populations with PEM prevalence rates of less than 5% had a mean CMR of 0.9/1,000/month. Refugee populations with PEM prevalences of greater than or equal to 40%, however, experienced a mean CMR of 37/1,000/month with a range of 4/1,000/month to 177/1,000/month ( Figure 7). The rate ratio between the lowest and highest CMR values was 40.7 (14). The close correlation between malnutrition prevalence and crude mortality during a relief operation for Somali refugees in eastern Ethiopia in 1988-1989 is clearly demonstrated in (Figure 8. Malnutrition prevalence was estimated by serial, cross-sectional, cluster sample surveys of children less than 5 years of age, and monthly death rates were estimated retrospectively by a population-based survey in August 1989. During the period of high malnutrition prevalence and high mortality (March-May 1989), food rations provided an average of approximately 1,400 kilocalories (kcal)/person/day instead of the recommended minimum of 1,900 kcal/person/day (9). Likewise, in eastern Sudan in 1985, inadequate amounts of food (1,360-1,870 kcal/person/day) were distributed to Ethiopian refugees during the first 5 months after their arrival in the camps. Malnutrition rates, as well as mortality rates, remained high during this period (Figure 3) ( Table 5). In addition, a severe measles outbreak in the Sudanese camps added to the high mortality (21).
Nutritional DiseasesProtein-energy malnutritionPEM can refer to either acute or chronic undernutrition. Because children less than 5 years of age are among the most acutely affected by undernutrition, assessment of this age group by anthropometry is usually done to determine PEM prevalence in a population (see "Indicators of Acute Undernutrition"). In general, acute undernutrition results in wasting and is assessed by an index of weight-for-height (WFH); however, edema of the extremities may be associated with acute undernutrition in which case, a clinical assessment is necessary. Chronic undernutrition produces stunting and typically results in a diminished height-for-age index. The prevalence of moderate to severe acute undernutrition in a random sample of children less than 5 years of age is generally a reliable indicator of this condition in a population. Since weight is more sensitive to sudden changes in food availability than height, nutritional assessments during emergencies focus on measuring WFH. Also, WFH is a more appropriate measurement for ongoing monitoring of the effectiveness of feeding programs. As a screening measurement, the mid-upper arm circumference (MUAC) may also be used to assess acute undernutrition, although there is not complete agreement on which cutoff values should be used as indicators. Nutritional assessment methods are fully described in the Rapid Nutrition Assessment Manual. * * Available from the International Health Program Office (IHPO), CDC, 1600 Clifton Road, MS F-03, Atlanta, GA 30333, 404-639-0308. Anthropometric indices such as WFH and height-for-age are interpreted by comparison with a "reference population". Index values are assigned a "Z-score" based on the number of standard deviations above or below the median value in the reference population. Currently, the World Health Organization (WHO) recommends the use of the CDC/NCHS reference population for nutritional assessments in all countries (22). Before the mid-1980's, anthropometric data was reported as a percentage of the median of the reference population value. Current international guidelines, however, recommend the use of Z-scores to report nutritional assessment data. Tables in this report define acute undernutrition on the basis of percentage median in order to allow comparisons of recent data with data from surveys performed before the mid-1980s. In a well-nourished population in which WFH values are distributed normally (i.e., the reference population), approximately 3% of children less than 5 years of age will have WFH Z-scores of less than -2. For less developed countries with lower "normal" nutritional intake levels, 5% of the children may have a Z-score less than -2 when compared with the reference population median, particularly at certain times of the year. Relief organizations agree that a nutritional emergency exists if greater than 8% of the children sampled have a Z-score less than -2. An excess of even 1% of children with Z-scores less than -3 indicates a need for immediate action. Acute PEM prevalence rates have been high in recent famine-affected populations, especially in Africa (Table 6). In addition, acute undernutrition prevalence rates have been elevated in many displaced and refugee populations during the past 12 years, ranging as high as 50% in eastern Sudan in 1985 (Table 5) and (Table 7). PEM rates have decreased rapidly in situations where effective emergency relief operations have been mounted promptly, i.e., Thailand (1979) and Pakistan (1980). However, in other emergencies, such as in Somalia (1980) and Sudan (1985), PEM rates have remained high (greater than 20%) for 6-8 months. Of even greater concern is the observation that acute undernutrition rates among Somali refugees in Ethiopia (1988-1989) actually increased 6 months after a relief program was launched. Although most high acute undernutrition prevalence has been associated with inadequate food rations, it appears that malnutrition developed among Kurdish children 1-2 years of age in Turkey within a period of 1-2 months, primarily because of the high incidence of diarrheal diseases in the camps (10). Among internally displaced civilian populations, high PEM prevalence has been associated with the intentional use of food as a weapon by competing military forces (30). The use of serial anthropometry surveys as monitoring tools has certain limitations when mortality rates are high. For example, an analysis of anthropometric data from two cross-sectional surveys in a refugee camp in Sudan in 1985 initially implied a relatively stable nutritional situation. In January, the prevalence of acute malnutrition in children less than 5 years of age was 26.3%; in March, the rate was 28.4%. During these two months, almost 13% of the children in the camp died, mainly from measles and diarrheal diseases. In this instance, the elevated child mortality rate masked diminished nutritional status in the population. Many malnourished children in the first survey, who had died, were "replaced" in the second survey sample by surviving children whose nutritional status had meanwhile deteriorated (31). Thus, anthropometry data need to be interpreted in the context of concurrent mortality rates. Micronutrient deficiency diseasesIn addition to PEM, micronutrient deficiencies play a key role in nutrition-related morbidity and mortality. The importance of micronutrient deficiencies in famine-affected and displaced populations has recently been extensively documented. In addition to deficiencies of vitamin A and iron, conditions widely recognized as important childhood problems in developing countries (i.e., epidemics of scurvy and pellagra) have also been reported in refugee populations during the past decade (Table 8). Vitamin A deficiencyThe most common deficiency syndrome in emergency affected populations is caused by lack of vitamin A. Ocular signs of vitamin A deficiency -- known as xerophthalmia -- include night blindness and Bitot's spots in the earlier stages. Xerophthalmia progresses to corneal xerosis, ulceration and scarring, and eventually blindness. Signs of xerophthalmia were detected in 7% of children surveyed in one region of Somalia during the drought of 1986-1987 (27); 2.1% in drought-affected Niger in 1985 (24); 4.3% among Kampuchean refugees in Thailand (36); and 2.7% in a region of Mauritania in 1984 (23). Recent data suggest that vitamin A deficiency is linked with high childhood mortality (37-38). Famine-affected and displaced populations often have low levels of dietary vitamin A intake before experiencing famine or displacement, and therefore, may have very low vitamin A reserves. Furthermore, the typical rations provided in large-scale relief operations lack vitamin A, putting these populations at high risk. In addition, some communicable diseases that are highly incident in refugee camps -- measles and diarrheal diseases -- rapidly deplete vitamin A stores. Depleted vitamin A stores need to be adequately replenished during recovery from these diseases to prevent the deficiency from becoming clinically important. Vitamin C deficiency (scurvy)Although scurvy has been reported rarely in stable populations in developing countries, many outbreaks have occurred in displaced and famine-affected populations in recent years, primarily because of inadequate vitamin C in rations. In 1981-1982, an outbreak of more than 2,000 cases of scurvy occurred in the refugee camps of the Gedo region of Somalia. These Ethiopian refugees had traditionally obtained sufficient dietary vitamin C from camel's milk. Once in refugee camps they subsisted on a ration devoid of vitamin C. The outbreak was precipitated when local markets, where refugees had exchanged rations for fresh fruit and vegetables, were suddenly closed (39). Active surveillance for scurvy among Ethiopian refugees in Somalia and Sudan in 1987 revealed cumulative incidence rates of up to 19.8% in some camps, with initial onset reported between 3-10 months after the arrival of the refugees (32). Cross-sectional surveys performed in 1986-1987 reported point prevalence rates as high as 45% among females and 36% among males; prevalence increased with age. The prevalence of scurvy was associated with the period of residence in camps, and the time exposed to rations lacking in vitamin C. In 1989, a population survey of children less than 5 years of age in Hartisheik camp in eastern Ethiopia in 1989 found the prevalence of clinical scurvy to be 2% (19). The international community has not developed an adequate strategy to prevent scurvy in refugee camps at the Horn of Africa, as demonstrated by an outbreak that took place among adult males (former Ethiopian soldiers) in a camp in eastern Sudan during 1991 (Bhatia R, personal communication, October 1991). Niacin deficiencyPellagra is the condition resulting from a severe deficiency of biologically available niacin in the diet. Once common in the southeastern United States, Italy, and Spain, pellagra now occurs mainly in maize- or sorghum-consuming populations in southern Africa, North Africa, and India. An outbreak of pellagra occurred in Malawi among Mozambican refugees between July and October 1989. Eleven camps reported a total of 1,169 patients; 20% of the patients were children less than 5 years of age (40). The French agency Medecins Sans Frontieres (MSF) instituted active surveillance at the time. Another outbreak occurred between February and October 1990 with 17,878 cases reported among 285,942 refugees in the same 11 sites (attack rate of 6.3%). More than 18,000 cases of deficiency were reported from all districts hosting approximately 900,000 refugees in southern Malawi, for an overall attack rate of 2.0% (35). Food rations contained an average of 4.9 mg of available niacin/person/day; the Food and Agriculture Organization (FAO)/WHO recommendations for daily niacin intake range from 5.4 mg for infants to 20.3 mg for adults. This outbreak occurred when relief efforts failed to include an adequate supply of groundnuts (peanuts), the major source of niacin in refugee rations. The lack of variety in basic relief rations is a major risk factor for pellagra and other micronutrient deficiency syndromes. Treatment of maize flour with lime (which converts niacin to a biologically available form of niacin) and the inclusion of beans, groundnuts, or fortified cereals in daily rations increase the total intake of available niacin and will prevent the development of pellagra (35). AnemiaThe high prevalence of anemia in refugee and displaced populations has been noted in few publications to date, but unpublished data from CDC assessments suggest that it may be a serious problem in some areas. In 1990, a survey of Palestinian refugees in Syria, Jordan, and the West Bank revealed that the prevalence of anemia among infants and young children was between 50% and 70%. Anemia among both nonpregnant and pregnant women was shown to be 25%-50%, whereas a low anemia prevalence rate was found among the male population. (In this study anemia was defined as a hemoglobin concentration of less than 11 g/dL among children and less than 12 g/dL among nonpregnant women. Pregnant women were considered to be anemic if their hemoglobin concentration was less than 11.5 g/dL during either the first or third trimester, or less than 11.0 g/dL during the second trimester.) These findings suggest that iron deficiency, which preferentially affects women and children, was the primary cause of anemia in this population. A 1987 study among refugees in Somalia demonstrated an anemia prevalence rate of 44%-71% among pregnant women, with that proportion being even greater if only women in the third trimester of pregnancy were considered. The cutoff point for hemoglobin concentration in this study was 10 g/dL; with the WHO cutoff of 11 g/dL, the prevalence would have been greater. Among children 9-36 months of age, 59%-90% were below the 10 g/dL cutoff. The inadequacy of the general ration was identified as the major factor causing iron deficiency anemia in this population. In a 1990 study, the prevalence rate of anemia was 13% among children less than 5 years of age in an Ethiopian camp for Somali refugees (Save the Children Fund UK, unpublished data). In addition to dietary iron deficiency, the high incidence of malaria in many refugee populations probably contributes to the high prevalence of anemia in children. This high prevalence of anemia found in some refugee populations may not be significantly greater than that found in local, non-refugee populations, since the latter group has been poorly documented. Nevertheless, anemia may be an additional important preventable risk factor for high mortality in refugee populations. The high prevalence of anemia is often correlated with a subset of the population with severe anemia (hemoglobin (Hb) less than 5 g). Severe anemia in itself can be a major cause of mortality for young children and pregnant women during the peripartum period. Other micronutrient deficienciesBeriberi (thiamine deficiency) has been reported from several refugee populations that subsist on rice-based food rations (Thailand, 1980; Guinea, 1990). Data regarding iodine deficiency in displaced populations are difficult to find, anecdotal evidence suggests that iodine deficiency, as evidenced by the presence of goiter, has been a problem in at least some camps in Pakistan and Ethiopia (CDC. Toole M, trip report, 1991).
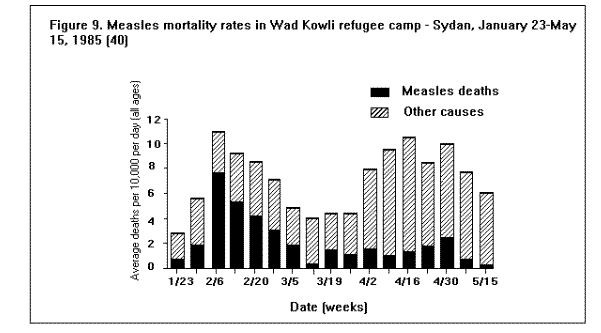
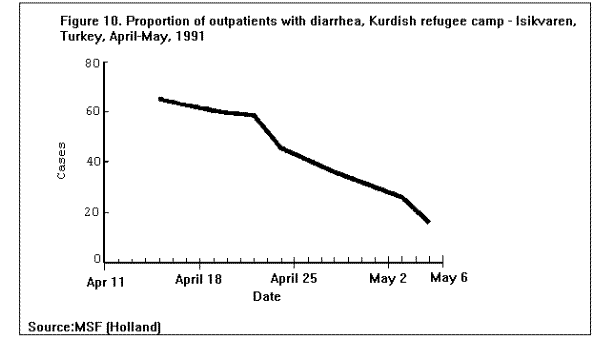
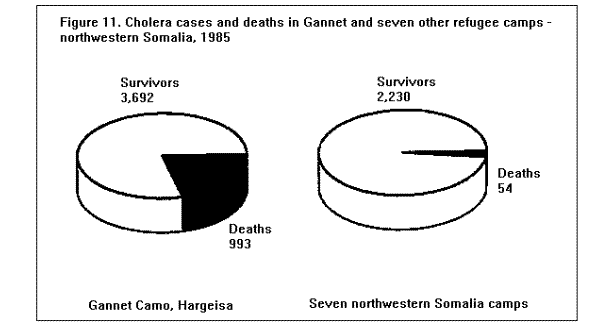
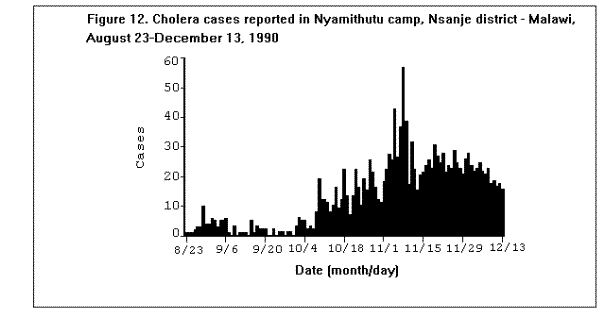
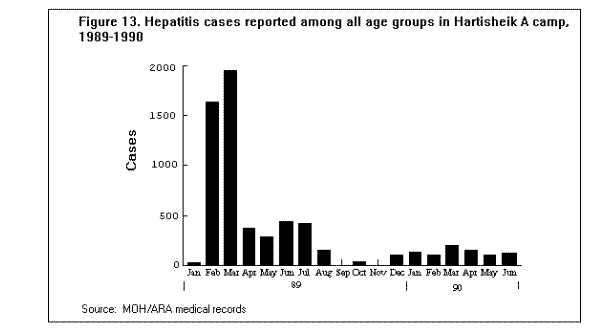
Communicable DiseasesMeasles, diarrheal diseases, ARIs, and in some cases, malaria are the primary causes of morbidity and mortality among refugee and displaced populations (1,16,41). (Figure 4), (Figure 5), and (Figure 6) illustrate patterns of mortality typical among those found in refugee camps. Other communicable diseases, i.e., meningococcal meningitis, hepatitis, typhoid fever, and relapsing fever have also been observed among refugee populations; however, the contribution of these illnesses to the overall burden of disease among refugees has been relatively small. Densely populated camps with poor sanitation, inadequate clean water supplies, and low-quality housing all contribute to the rapid spread of disease in refugee settings. In addition, the interaction between malnutrition and infection in these populations, particularly among young children, has contributed to the high rates of morbidity and mortality from communicable diseases. Available and affordable technology could prevent much of this morbidity and mortality either through primary prevention (e.g., immunization, adequate planning, and sanitation) or through appropriate case management (e.g., treatment of dehydration caused by diarrhea with oral rehydration salts and continued feeding). MeaslesOutbreaks of measles within refugee camps have been common and have caused many deaths. Low levels of immunization coverage, coupled with high rates of undernutrition and vitamin A deficiency, have played a critical role in the spread of measles and the subsequent mortality within some refugee camps. Measles has been one of the leading causes of death among children in refugee camps. In addition, measles has contributed to high malnutrition rates among those who have survived the initial illness. Measles infection may lead to or exacerbate vitamin A deficiency, compromising immunity and leaving the patient susceptible to xerophthalmia, blindness, and premature death (42). In early 1985, the crude, measles-specific death rate in one eastern Sudan camp reached 13/1,000/month; among children less than 5 years of age, the measles-specific death rate was 30/1,000/month. Over 2,000 measles deaths were reported in this camp from February through May 1985. (Figure 9) illustrates the proportion of all deaths that were due to measles in this camp during the course of the outbreak (16). The CFR was reported to be 33% during this outbreak; however, mild cases may have been underreported. Large numbers of measles deaths have been reported in camps in Somalia, Bangladesh, Sudan, and Ethiopia (1). Mass immunization campaigns were effective in reducing the measles morbidity and mortality rates in camps in both Somalia and Thailand (16). Measles outbreaks probably did not occur during certain other major refugee emergencies (e.g., Somalis in Ethiopia in 1989; Iraqis in Turkey in 1991), because immunization coverage rates were already high in those refugee populations before their flight (9,10). Diarrheal diseasesDiarrheal diseases are a major cause of morbidity and mortality among refugee and displaced populations, primarily because of the inadequacy of the water supply (both in terms of quality and quantity), and the insufficient and poorly maintained sanitation facilities. In eastern Sudan in 1985, between 25%-50% of all deaths in four major camps were attributed to diarrheal diseases. In Somalia (1980), Malawi (1988), and Ethiopia (1989), between 28%-40% of all deaths in refugee camps were attributed to diarrhea (1). Between March and October 1991, 35% of deaths among Somali refugees in the Liboi camp in Kenya were caused by diarrhea. Among Central American refugees in Honduras, diarrheal diseases were responsible for 22.3% of mortality among children less than 5 years of age during a 3-year period (43). In April 1991, in camps for Iraqi refugees on the Turkish border, approximately 70% of all patients arriving at clinics had diarrhea (10). Of these, approximately 25% complained of bloody diarrhea during the first 2 weeks of April. (Figure 10 ) shows the gradual decline in diarrheal disease among clinic outpatients at a Kurdish refugee camp in Turkey. Improvements in camp sanitation and water supply were probably responsible for this trend. Although the etiologies of diarrheal illness during refugee emergencies have not been well documented, the responsible pathogens are most likely to be the same agents that cause diarrhea in non-refugee populations in developing countries. In one study in a camp for famine victims in Ethiopia, of 200 patients with diarrhea, 15.6% had positive cultures for Escherichia coli (pathogenicity not specified by authors), 3.5% for Shigella spp., and 2% for Salmonella spp. (44). CholeraOutbreaks of cholera have occurred in several refugee populations, although overall, other diarrheal diseases have probably caused many more deaths than cholera. In addition to the morbidity and mortality directly caused by cholera, epidemics of this severe disease cause serious disruption to camp health services. Outbreaks of cholera have been reported in refugee camps in Thailand (16,45), Sudan (46), Ethiopia (11-12), Malawi (47), Somalia (48), and Turkey (10). The Somali Refugee Health Unit reported 6,560 cases of cholera and 1,069 cholera deaths in 1985. During the course of the epidemic, one camp (Gannet) experienced a CFR of 25%. The CFR in the remaining camps was 2.9%, with some areas reporting a CFR of less than 1% (Figure 11) (48). During the same year, two adjacent refugee camps in the Sudan reported a total of 1,175 cases of cholera with 51 deaths (CFR = 4%) over the course of a 2-week epidemic (46). Mozambican refugees in Malawi have been especially vulnerable to cholera; 20 separate outbreaks have been reported in Malawian camps since 1988 (49). Outbreak investigations have identified polluted water sources, shared water containers and cooking pots, lack of soap, failure to reheat leftover food, and possibly contaminated food (dried fish) as important risk factors for infection. Nearly 2,000 cases were reported among 80,000 refugees in one camp (Nyamithutu) during a 4-month period in 1990 ( Figure 12). Among 26,165 new arrivals during this period, 1,651 cases were reported for an attack rate of 6.3% in this group. The variation in CFRs between camps reflects the different levels of organizational preparedness, health worker training and experience, and available resources. One group of relief workers speculated that high CFRs in some Malawian camps may be associated with concurrent niacin deficiency, although their hypothesis has not yet been proven (Moren A, personal communication). Acute respiratory infectionsARIs are among the leading causes of death among refugee populations. In Thailand (1979), Somalia (1980), Sudan (1985), and Honduras (1984-1987), ARIs were cited among the three main causes of mortality in refugee camps, particularly among children (16,43). Among children less than 5 years of age in refugee camps in Honduras, respiratory infections were responsible for slightly greater than 1 of every 5 deaths during a 3-year period (43). Tuberculosis (TB)TB is well recognized as a health problem among refugee populations. The crowded living conditions and underlying poor nutritional status of refugee populations may foster the spread of the disease. Although not a leading cause of mortality during the emergency phase, TB often emerges as a critical problem once measles and diarrheal diseases have been adequately controlled. For example, 26% of adult deaths among refugees in Somalia in 1985 were attributed to TB (16). During this time, TB was the third leading cause of death, and the leading cause among adults (48). In eastern Sudan, between 38% and 50% of all deaths in two camps were caused by TB during the 9 to 10 months period after the camps opened (16). TB has been cited as a major health problem among Afghan refugees in Pakistan (CDC. Serdula M, trip report). Although it may be theoretically easier to ensure patient compliance with protracted chemotherapy in the confined space of a refugee camp, the personnel needed to supervise treatment may not be available. In addition, the uncertain duration of stay, frequent changes of camp locations, and poor camp organization may hinder TB treatment programs. MalariaMalaria is a major health problem in many areas that host large refugee populations, including Somalia, Sudan, Ethiopia, Thailand, Guinea, Cote d'Ivoire, Malawi, Pakistan, and Kenya. Malnutrition and anemia, conditions that are common among refugees, may be directly related to recurrent or persistent malaria infection or may compound the effects of malaria and lead to high mortality. Malaria is the leading cause of morbidity among adult refugees in Malawi and in 1990 caused 18% of all deaths and 25% of deaths among children less than 5 years of age (CDC, unpublished data). Malaria is of particular concern when the displaced population has traveled through, or into, an area of higher endemicity than its region of origin (1). During the period 1979- 1980, Khmer refugees traveled from the central valley of Kampuchea, where malaria transmission is very low, into Thailand. Those refugees who arrived at the Sakaeo camp traveled through mountain regions where malaria is highly endemic year round, while refugees who arrived at Khao I-Dang camp had traveled a route that remained within the areas of low malaria transmission. As a result of the differences in exposure during transit, the initial malaria prevalence rate at Sakaeo was 39% compared with a 4% prevalence rate at Khao I-Dang. During this time, malaria was a major cause of death at Sakaeo (50). Similarly, Ethiopian refugees from the highland areas of Tigray province arrived in eastern Sudan in 1985 with decreased immunity against the malaria that is seasonally endemic in that region of Sudan. Not surprisingly, malaria was an important cause of death among these refugees. Farther north, in the Kassala region of eastern Sudan, a major outbreak of malaria occurred among refugees from Eritrea following extensive flooding in the area in September 1988. In contrast to the Tigrayan refugees, the Eritreans were largely from lowland areas and had been previously exposed to malaria. The severity of this outbreak may have been due to the emergence of chloroquine resistant Plasmodium falciparum malaria in eastern Sudan at that time, and the subsequent widespread failure of first-line treatment regimens (Toussie S, personal communication, 1989). Afghan refugees living in the North-West Frontier Province of Pakistan have a higher incidence of clinical malaria than that observed among the local population. A comparison of the epidemiologic trends of malaria between the refugees and the local population over a period of several years demonstrated that the increased rate of malaria illness among refugees was a result of having resettled in an area of higher transmission than that from which they had fled. Because of their limited exposure history, the Afghan refugees had lower levels of immunity to malaria illness than did the local population (51). Few deaths associated with malaria have been reported in this population because the majority of cases have been associated with Plasmodium vivax, a milder form of malaria than that caused by Plasmodium falciparum, the form that is more commonly reported in African camps. HepatitisHepatitis has not been among the most common diseases reported in refugee and displaced populations worldwide, however, since 1985 it has emerged as a serious problem in camps at the Horn of Africa, where access to adequate supplies of clean water has been severely limited. In Somalia during the period 1985-1986, an outbreak of greater than 2,000 cases occurred in two refugee camps, with an overall attack rate of 8% among adults. Of 87 hepatitis deaths, 46% were among pregnant women. The overall CFR was 4%, the CFR in second- and third-trimester pregnant women was 17%. By a process of exclusion, the outbreak was attributed to enterically transmitted non-A, non-B hepatitis (now known as hepatitis E) (52). (Figure 13) depicts an outbreak of hepatitis that occurred in the Hartisheik refugee camp in Ethiopia between 1989 and 1990. During an 18-month period, greater than 6,000 cases were reported. Between March and October of 1991, a major outbreak of hepatitis occurred among Somali refugees living in Kenya's Liboi camp; a total of 1,700 cases were reported, yielding an attack rate of 6.3%. The overall CFR was 3.7% and in pregnant women the CFR was 14%. Hepatitis was responsible for one of every five deaths in the camp during that time period. The hepatitis E virus was identified in stool and serum specimens from ill patients. The Ethiopian and Kenyan outbreaks were associated with inadequate water supply. In both camps, refugees had access to an average of only 1-3 liters of clean water/person/day (the United Nations High Commissioner for Refugees (UNHCR) recommends a minimum of 15 liters/person/day) (53). MeningitisOvercrowding and limited access to medical care are contributing factors in outbreaks of meningococcal meningitis among refugee populations. Also, many large refugee populations are found in what is termed the "meningitis belt" of sub-Saharan Africa. Although children less than 5 years of age are at greatest risk for meningitis, meningococcal meningitis also occurs among older children and adults, particularly in densely populated settings i.e., refugee camps (54). During an outbreak of group A meningococcal disease at the Sakaeo refugee camp in Thailand in 1980, children less than 5 years of age experienced a CFR of 50%. The overall CFR during that outbreak was just over 28% (55). Outbreaks of meningococcal meningitis have also been reported among Ethiopian refugees in eastern Sudan (1985) and among displaced Sudanese in Khartoum and southern Sudan during 1988 (56).
Other Health IssuesAlthough these reports focus on the major causes of morbidity and mortality during the emergency phase of refugee displacements, other health problems warrant the attention of public health practitioners in these settings. InjuriesThus far, injuries related to armed conflict and psychological problems relating to war, persecution, and the flight of the refugee have been poorly quantified. In a recent report on Iraqi refugees on the Turkish border, 8% of the deaths during a 2-month period were attributed to trauma. Sixty percent of these trauma-related deaths were attributable to shootings by armed soldiers (CDC. Toole M, trip report, September 1991). Anecdotal reports support the existence of high rates of physical disabilities caused by war injuries in some refugee camps, such as those for Afghan refugees in Pakistan, Cambodian refugees in Thailand, and Mozambican refugees in Malawi. Maternal healthThe problem of morbidity and mortality related to pregnancy and childbirth has been inadequately documented, although earlier sections of this report described high anemia rates and high hepatitis-specific mortality rates among pregnant women (52). Also, studies of scurvy and pellagra among refugees in Africa have consistently revealed higher incidence rates in women than in men, and a study in Somalia showed that pregnancy was a risk factor for the development of clinical scurvy (32,35). Sexually transmitted diseases and HIVFew published reports have referred to sexually transmitted diseases (STD) in refugee populations. However, there is no evidence that the incidence of STDs in camps is any higher (or lower) than in non-refugee communities. Similarly, practically no data exist on the prevalence of HIV infection, nor on rates of transmission in these populations. Many of the large displaced and refugee populations of the world are either located in, or have fled to, countries where HIV prevalence rates are high. These include: Mozambican refugees in Malawi, Zambia, and South Africa; Ethiopian refugees in Sudan; Liberian refugees in Cote d'Ivoire and Guinea; Ugandan and Rwandan refugees in Zaire; Cambodian and Laotian refugees in Thailand; and Sudanese refugees in Uganda.
REFERENCES
RECOMMENDATIONSThe technical recommendations in this report focus on the public health elements of an appropriate response program for refugees and displaced persons, however, the effectiveness of relief efforts will be enhanced if the affected communities and host countries have prepared for the emergency. Preparedness for sudden population displacement is critical and should be targeted at the most important public health problems identified in previous emergencies: malnutrition, measles, diarrheal diseases, malaria, ARI, and other communicable diseases (e.g., meningitis and hepatitis) that result in high death rates. Preparedness requires that planning for emergencies be included as an integral part of routine health development programs in countries where sudden population displacements might occur. These programs include:
National public health programs should include detailed contingency planning for sudden population movements, both internally and from neighboring countries.
Response PreparednessThe critical components of a relief program responding to sudden population displacement comprise the provision of adequate food, clean water, sanitation, and shelter. In addition, the following elements of a health program should be established as soon as possible. Health Information System
Diarrheal Disease Control
Immunization
Basic Curative Care
Endemic disease control and epidemic preparedness
The detailed recommendations that follow are organized according to either disease group (e.g., diarrheal diseases or malnutrition) or technical methods (e.g., rapid assessment). Nevertheless, it is critical to keep in mind the demographic groups that are most at risk during emergencies, namely young children and women. It is important that health services in refugee settings be organized in a way that facilitates access by these groups. In general, MCH services should be given higher priority than general outpatient dispensaries and hospitals.
Maternal and Child Health CareMCH clinics should be established (ideally one MCH clinic per 5,000 population) and staffed by trained personnel to provide routine screening and preventive, and curative services to pregnant and lactating women and to children less than 2 years of age. If resources are adequate, these services should be extended to children between 2 and 5 years of age. Services for children should include routine growth monitoring, immunization, nutritional rehabilitation, vitamin A supplementation, and curative care, as well as health education for their mothers. Female health workers should be trained and employed to provide culturally appropriate health education both at MCH clinics and within the community, and to refer pregnant women to the clinic for antenatal care. At least some of these health workers should be recruited from among traditional birth attendants in the community. Antenatal care should include screening for high-risk pregnancies and providing iron and folic acid supplementation (as well as iodine supplementation in areas of endemic goiter), tetanus toxoid immunization, and health education. Postnatal care should include nutritional supplementation, counselling on family spacing, provision of contraceptives, and education about breastfeeding and infant care. In certain cultural situations, curative care may need to be provided to all women of child-bearing age in a setting physically segregated from male outpatient facilities.
Program-Specific RecommendationsThe following content areas are covered in these recommendations:
Rapid Health AssessmentRapid health assessment of an acute population displacement is conducted to:
PreparationsThe amount of time required to conduct an initial assessment of a refugee influx depends on the remoteness of the location, availability of transport, security situation in the area, availability of appropriate specialists, and willingness of the host country government to involve external agencies in refugee relief programs. In small countries with functioning communications facilities and secure borders, the assessment might be conducted in 4 days; in other countries, it might take 2 weeks. Before the field visit, relevant information relating to the status of the incoming refugees, as well as the available resources of the host community, should be obtained from local ministries or organizations based in the capital city. Any maps of the area where the refugees are arriving and settling should likewise be obtained. Aerial photographs will also be of value, but may be considered sensitive by the military of the host country. International organizations like UNICEF, WHO, and the Red Cross/Red Crescent may also have demographic and health data concerning the refugee population. In preparation for the field visit, establish whether food, medical supplies (including vaccines), or other relief supplies have been ordered or procured by any of the relief agencies involved. Additionally, the following conditions should be included in a field assessment. Field assessmentThe following demographic information is required to determine the health status of the population.
Why this information is needed. The total population will be used as the denominator for all birth, death, injury, morbidity, and malnutrition rates to be estimated later. The total population is necessary for the calculation of quantities of relief supplies. The breakdown of the population by age and sex allows for the calculation of age- and sex-specific rates and enables interventions to be targeted effectively (e.g., immunization campaigns). Sources of information. Local government officials or camp authorities may be able to provide registration records. If no registration system is in effect, one should be established immediately. Information recorded should include the names of household heads, the number of family members by age and sex, former village and region of residence, and ethnic group, if applicable. Refugee leaders may also have records, particularly if entire villages have fled together. In certain situations, political groups may have organized the exodus and may have detailed lists of refugee families. A visual inspection of the settlement may provide a general impression of the demographic composition of the population. However, information obtained in this manner should be used judiciously as it is likely to provide a distorted view of the situation. It may be necessary to conduct a limited survey on a convenience sample in order to obtain demographic information. Beginning at a randomly selected point, survey a sample (e.g., 50) of dwellings. Visit every fifth or 10th house until the predetermined number of houses have been surveyed. At each house, record the number of family members, the age and sex of each person, and the number of pregnant or lactating women. This process will establish an initial estimate of the demographic composition of the population. Estimate the number of persons in each house, as well as the total number of houses in the settlement, to gain a provisional estimate of the camp population. At the very least, this quick survey should give a rough estimate of the proportion of the total population made up of "vulnerable" groups; i.e., children less than 5 years of age and women of child bearing age. To determine the total population, a census may need to be conducted later. Background health informationThe information required includes:
Why this information is needed. Effective planning of health services will depend on this information. Planners need to be aware of traditional beliefs, taboos, and practices in order to avoid making costly mistakes and alienating the population. Sources of information. Obtain documents and reports from the host government, international organizations, and nongovernment organizations pertaining to endemic diseases and public health programs in the displaced population's region of origin. Interview refugee leaders, heads of households, women leaders (e.g., traditional midwives), and health workers among the refugee population. Seek information from development agencies, private companies, missionaries, or other groups having experience with the displaced population. Nutritional statusThe information required includes:
Why this information is needed. Evidence exists to support the fact that the nutritional status of displaced populations is closely linked with their chances of survival. Initial assessment of nutritional status serves to establish the degree of urgency in delivering food rations, the need for immediate supplementary feeding programs (SFPs), and the presence of micronutrient deficiencies that require urgent attention. Sources of nutritional information. If refugees are still arriving at the site:
If refugees are already located in a settlement:
In order to gather baseline data for evaluation of nutrition programs, plan to conduct a valid, cluster sample survey of the population as soon as possible (within 2 weeks). Appropriate technical expertise will be needed for the implementation and analysis of the survey. Mortality ratesThe information required includes crude, age-, sex-, and cause-specific mortality rates. Why this information is needed. In the initial stages of a population displacement, mortality rates, expressed as deaths/10,000/day, are a critical indicator of improving or deteriorating health status. In many African countries, the daily CMR (extrapolated from published annual rates) is approximately 0.5/10,000/day during non-emergency conditions. In general, health workers should be extremely concerned when CMRs in a displaced population exceed 1/10,000/day, or when less than 5 years of age mortality rates exceed 4/10,000/day. Sources of mortality information. Check local hospital records and the records of local burial contractors. Interview community leaders. Establish a mortality surveillance system. One approach is to designate a single burial site for the camp, which should be monitored by 24-hour grave watchers. Grave watchers should be trained to interview families, using a standard questionnaire, and then to record the data to determine gender, approximate age, and probable cause of death. Other methods of collecting mortality data include registering deaths, issuing burial shrouds to families of the deceased to ensure compliance, or employing volunteer community informants who report deaths for a defined section of the population. Demographic data are absolutely essential for calculating mortality rates. These provide the denominator for estimating death rates in the entire population and within specific vulnerable groups, such as children less than 5 years of age. The population needs to be assured that death registration will have no adverse consequences (e.g., ration reductions). Morbidity The information required includes age- and sex-specific data regarding the incidence of common diseases of public health importance, i.e., measles, malaria, diarrheal diseases, and ARI, as well as diseases of epidemic potential such as hepatitis and meningitis. The data should be collected by all health facilities, including feeding centers. Why this information is needed. Data on diseases of public health importance may help plan an effective preventive and curative health program for refugees. These data will also facilitate the procurement of appropriate medical supplies and the recruitment and training of appropriate medical personnel, as well as focus environmental sanitation efforts (e.g., toward mosquito control in areas of high malaria prevalence). Sources of morbidity information. Review the records of local clinics and hospitals to which refugees have access. Where a clinic, hospital, or feeding center has already been established within the camp, examine patient records or registers and tally common causes of morbidity. Interview refugee leaders and health workers within the refugee population. A simple morbidity surveillance system should be established as soon as curative services are established in the camp. Feeding centers should be included in the surveillance system. Community health workers should be trained as soon as possible to report diseases at the community level. The initiation of certain public health actions should not be delayed until the disease appears. For example, measles immunization should be implemented immediately. Do not wait for the appearance of measles in the camp. Also, oral rehydration centers should be routinely established in all situations. Environmental conditionsThe information required includes:
Why this information is needed. Information on local environmental conditions affecting the health of displaced populations will help relief planners create priorities for public health programs. Sources of information. This assessment is made largely by visual inspection. In addition, interviews with local government and technical specialists will yield important information. In some cases, special surveys need to be conducted; e.g., entomologists may need to survey for local disease vectors, and water engineers may need to assess water sources. Resources availableFood supplies -- Efforts to evaluate food supplies should include:
Food sources. Local, regional, and national markets need to be assessed. The cash and material resources of the displaced population should also be assessed in order to estimate its local purchasing power. Food logistics. Assess transport and fuel availability, storage facilities (size, security), and seasonal conditions of access roads. Feeding programs. Follow these guidelines to evaluate feeding programs:
Local health services. Follow these guidelines for assessing the capabilities of health services:
Camp health services. Follow these guidelines for assessing camp health services:
Taking action
Checklist For Rapid Health Assessment (*) (*) Adapted from : WHO Emergency Relief Operations. Emergency Preparedness and Response: Rapid Health Assessment in Sudden Population Displacements. WHO, in collaboration with CDC and other WHO Collaborating Centers for Emergency Preparedness and Response. Geneva: January 1990. Preparation
Field assessment
Health information
Nutritional status
Mortality rates
Morbidity
Environmental conditions
Resources available
Health Information SystemA health information system (HIS) provides continuous information on the health status of the refugee community and comprises both ongoing routine surveillance and intermittent population-based sample surveys. This information may be used to:
Data collectionAs soon as health services are established for a refugee population, a surveillance system should be instituted and should ideally be set up at the time of an initial, rapid assessment. Any agency or facility (including feeding centers) providing health services to the refugee population should be part of the reporting network. Any host community services to which the refugees might have access should also be part of the system. Health information should be reported on a simple, standardized surveillance form. (A sample form, adapted from WHO Emergency Relief Operations, is located at the end of this section.) Each health facility should be held accountable for completing the reporting form at the appropriate interval and for returning it to the person or agency charged with compiling the reports, analyzing the information, and providing feedback. Each refugee settlement or camp should have a person responsible for coordinating the HIS. Forms should be translated into the appropriate local language(s) if community health workers are involved in information collection. Health facilities should keep a daily record of patients; age, sex, clinical and laboratory diagnosis, and treatment should be specified. If personnel time is limited, a simple tally sheet should be used. In addition, the patient should be issued a health record card on which the date, diagnosis, and treatment are recorded. Each time a patient contacts the health-care system, whether for curative or preventive services, this should be noted on the health record card. Laboratory data should accompany diagnostic information whenever possible. Collecting Processing, Storing, and Shipping Diagnostic Specimens in Refugee Health-Care Environments * provides an overview of procedures for collecting and processing diagnostic specimens in the field. * Available from IHPO, CDC, 1600 Clifton Road, MS F-03, Atlanta, GA 30333, 404-639-0308. Data collection should be limited to that information that can and will be acted upon. Information that is not immediately useful should not be collected during the emergency phase of a refugee relief operation. Overly detailed or complex reporting requirements will result in noncompliance. The most valuable data are generally simple to collect and to analyze. Standard case definitions for the most common causes of morbidity and mortality should be developed and put in writing. The data collected will fall into one of the following categories: a) demographic, b) mortality, c) morbidity, d) nutritional status, and e) health program activities. Population. Camp registration records should provide most of the demographic information needed. If registration records are inadequate, a population census may be necessary. Conducting a census is often politically sensitive and may be delayed by the administrative authorities for a long period of time. Consequently, innovative methods may need to be devised. For example, organize a nutritional screening of all children less than 5 years of age. Count the children and estimate the percentage of the total population less than 5 years of age by doing a sample survey. From this information, estimate the total population size. For other methods to determine population size and structure see "Rapid Health Assessment". It is important that population figures be updated on a regular basis, taking into account new arrivals, departures, births, and deaths. The total population is used as the denominator in the calculation of disease incidence, birth, and death rates. This total is also necessary to determine requirements for food and medical supplies and to estimate program coverage rates. Information about the population structure is needed to calculate age- and sex-specific morbidity and mortality rates, to estimate ration requirements, and to determine the target population for specific interventions, i.e., antenatal care and immunizations. The rate of new arrivals and departures gives an indication of the stability of the population and will influence policy decisions about long-term interventions, such as TB therapy. This information is also used to predict future resource and program needs. A birth registration system is usually simple, since the community expects an increase in the family food ration as a result of a new birth. Births might be reported in the community to volunteer health workers or traditional birth attendants. Alternatively, if good antenatal care services are established, follow-up of pregnant mothers will allow for a relatively complete registration of births. Examples of mortality surveillance systems are described in "Rapid Health Assessment". Deaths may be underreported if there is a fear of possible ration reduction; thus, an agreement might be negotiated with camp authorities not to decrease rations after a death occurs at least during the emergency phase. Arrivals and departures should be monitored through the camp registration system. Mortality. Each health facility should keep a log of all patient deaths (with cause of death and relevant demographic information) and report the deaths on a standardized form. Because many deaths occur outside of the health-care system, a community-based mortality surveillance system should be established. Such a system may include the employment of grave watchers, the routine issuance of burial shrouds, and the use of community informants (see "Rapid Health Assessment"). Death rates are the most specific indicators of a population's health status and are the category of data to which donors and relief agencies most readily respond. During the emergency phase of a relief operation, death rates should be expressed as deaths/10,000/day to allow for detection of sudden changes. In refugee camps, relief programs should aim at achieving a CMR of less than 1/10,000/day as soon as possible. This rate still represents approximately twice the "normal" CMR for non-displaced populations in most developing nations and should not signal a relaxation of efforts. After the emergency phase, death rates should be expressed as deaths/1,000/month to reflect the usual reporting frequency and to facilitate comparison with baseline, non-refugee death rates. Age- and sex-specific mortality rates will indicate the need for interventions targeted at specific vulnerable groups. During the early stage of a relief operation, specific death rates for persons less than 5 years of age and greater than 5 years of age may suffice. Later, further disaggregation by age may be feasible -- for example, less than 1 year, 1-4 years, 5-14 years, and greater than 15 years. Different male- and female-specific death rates may reflect inequitable access to resources or health services. Cause-specific mortality rates will reflect those health problems having the greatest impact on the refugee community and requiring the highest priority in public health program planning. Morbidity. Health facilities and feeding centers should report morbidity information on the same form on which mortality is reported. Each disease reported in the system must have a written case definition that will guide health workers in their diagnosis and ensure the validity of data. Where practical, case definitions that rely on clinical signs and symptoms should be tested periodically for sensitivity and specificity as compared with a laboratory standard (e.g., malaria). Knowledge of the major causes of illness and the groups in the affected population that are at greatest risk allows for the efficient planning of intervention strategies and the most effective use of resources. Morbidity rates are more useful than a simple tallying of cases, as trends can be followed over time, or rates compared with those from different populations. The monitoring of proportional morbidity (e.g., percentage of all morbidity caused by specific diseases) may be useful when specific control measures are being evaluated, although caution is needed in the interpretation of trends. A relative decrease in disease-specific proportional morbidity may merely reflect an absolute increase in the incidence of another disease. Nutritional status. Data regarding nutritional status can be obtained through a nutritional assessment survey or a mass screening exercise. Surveys should be repeated at regular intervals to determine changes in nutritional status; however, not so frequently as to obscure true differences between surveys. All children less than 5 years of age should undergo a nutritional screening upon arrival at the camp and should continue to be weighed and measured monthly at MCH clinics in the camp. Information collected during these screenings should be included in HIS reports. If the initial screening identifies high prevalence rates of undernutrition, cross-sectional surveys should be repeated at intervals of 6-8 weeks until the undernutrition prevalence rate is below 10%. Thereafter, surveys every 6-12 months will suffice, unless routine surveillance data indicate that nutritional status has deteriorated. Measurement of nutritional status is described in the Rapid Nutrition Assessment Manual. (*) (*) Available from IHPO, CDC, 1600 Clifton Road, MS F-03, Atlanta, GA 30333, 404-639-0308. The prevalence of acute malnutrition acts as an indicator of the adequacy of the relief ration. A high prevalence of malnutrition in the presence of an adequate average daily ration may indicate inequities in the food distribution system, or high incidence rates of communicable diseases (e.g., measles and diarrhea). The presence of nutritional deficiency disorders (i.e., pellagra, anemia, or xerophthalmia) indicates the need for ration supplementation. Programs. Each health facility should keep a log of all activities. Immunizations should be recorded in a central record, as well as on the person's health record card. Records of health sector activities will be useful in determining whether certain groups in the population are underserved, and in planning measures to reach a broader population base. Although approximate immunization coverage may be estimated from the number of vaccine doses administered, the preferred method is by annual population surveys. Analysis and interpretationMost data can be analyzed locally using a pen and paper. The use of computers and a data entry and analysis program, such as Epi Info, version 5, may be practical at the regional or national level. Trends in mortality, morbidity, and nutritional status should be monitored closely. Careful attention should be paid to changing denominators, and changes in proportional mortality or morbidity should be interpreted with particular caution. Where applicable, correlations between mortality, morbidity or nutritional status, and health sector activities should be examined. Likewise, the proportion of malnourished children identified in population surveys as enrolled in feeding programs can be used to estimate program coverage. All components of the HIS should be analyzed and interpreted in an integrated fashion. A single element examined alone will reveal only a small portion of the entire picture and may be easily misinterpreted. For example, an apparent decrease in malnutrition prevalence should be interpreted in the context of childhood mortality rates (1). The use of health information to guide program decision-making will be facilitated if targets and critical indicators are established at the beginning. For example, a measles incidence rate of 1/1,000/month might be an indicator that would initiate specific preventive actions. Similarly, during a cholera outbreak, a CFR of 3% in a given week might stimulate a critical review of case management procedures. Control measuresThe information gathered through the HIS should be used to develop recommendations and to implement specific control measures. Objectives for disease control programs should be established and progress towards these objectives regularly assessed. The presentation of data to decision-makers should make use of simple, clear tables and graphs. Most importantly, there should be regular feedback to the data providers through newsletters, bulletins, and frequent supervisory visits. AssessmentThe HIS should be periodically assessed to determine its accuracy, completeness, simplicity, flexibility, and timeliness. The utilization of the data by program planners and key decision-makers should also be assessed. The HIS should evolve as the need for information changes. Reference
NutritionRationsFor populations totally dependent upon food aid, a general ration of at least 1,900 kcal/person/day is required. At least 10% of the calories in the general ration should be in the form of fats and at least 12% should be derived from proteins.
The calculation of rations should account for calorie loss during transport and food preparation. Similarly, when the mean daily temperature falls below 20 C, the caloric requirement should be increased accordingly by 1% per degree of temperature below 20 C. The standard requirement of 1,900 kcals is based on the following demographic structure of a population:
The calculation of ration requirements should be adjusted for deviations from the above population structure (age/gender breakdown), the underlying health and nutritional status of the population, and relative activity levels of the community. Guidelines for ration distribution
Supplementary feeding programs SFPs are designed to help prevent severe malnutrition and to rehabilitate moderately malnourished persons. SFPs are not intended to be used as a method of targeting food during an emergency phase. Similarly, SFPs are inappropriate as a long-term supplement to an inadequate general ration. Implementation of a SFP is necessary under the following circumstances:
Inclusion and discharge criteria. The following groups should be targeted for inclusion in a SFP:
Children should be discharged from the SFP after they have maintained greater than 85% of median WFH (or a Z-score greater than -1.5) for a period of 1 month. Caloric requirements. A SFP should provide at least 500 kcal and 15 g protein/day in one or two feedings.
High energy milk (HEM), a calorie-dense milk mixture, may be used in a SFP.
One milliliter of HEM provides 1 kcal of energy. The formula below makes 5 L
of HEM: If the general ration is inadequate (less than 1,900 kcal/person/day), the supplementary ration should provide 700-1,000 kcal/person/day in two to three feedings. Types of SFPs. SFPs fall into two categories, either on-site feeding or take-home rations. Listed below are some of the advantages and disadvantages of each type of SFP (1). On-site feeding. "Wet" rations are prepared by SFP staff and served to recipients in the feeding center. Listed below are the advantages of wet rations:
These are the disadvantages of wet rations:
Take-home programs. "Dry" rations are provided on a regular basis to supplement the general ration normally received. These are the advantages of dry rations:
These are the disadvantages of dry rations:
Other elements of SFPs
Therapeutic feeding programs Therapeutic feeding programs (TFPs) are considered a medical intervention, the purpose of which is to save lives and restore the nutritional health of severely malnourished children. The recommendations listed below are adapted from the procedures for selective feeding (2). Enrollment criteria. Children should be enrolled in a TFP if they meet one of the following criteria:
Caloric requirements
Discharge criteria. Discharge from a TFP to a SFP should occur when the following criteria are met:
Monitoring requirements
Provision of micronutrients Ideally, the recommended daily allowances for all essential nutrients should be provided in the general rations. However, specific measures may be necessary to provide certain micronutrients. Vitamin A Risk factors for vitamin A deficiency. Provide vitamin A supplements whenever any of the following conditions are present:
Supplemental doses and schedule
In all cases, mothers should be administered 200,000 IU within 2 months of giving birth in order to provide adequate quantities of vitamin A in the breast milk. If it is not possible to provide supplements to the mother at or within 2 months of giving birth, then the mother should receive 100,000 IU during the third trimester of pregnancy.
Full treatment schedule. A full treatment schedule of oral vitamin A should
be administered to all persons suffering from severe malnutrition (WFH
Z-score less than -3) or exhibiting eye symptoms of vitamin A deficiency
(xerosis, Bitot's spots, keratomalacia, or corneal ulceration). The dose
schedule is given below: Anemia. The prevalence of anemia can be determined through a rapid anemia survey using a portable Hb photometer (HemoCue system). The CDC has established the following criteria for defining anemia:
The risk of anemia is highest in pregnant and lactating women, and in children ages 9-36 months. If the general ration contains inadequate amounts of absorbable iron, folate, and vitamin C, anemia may be prevented through the daily administration of iron/folate tablets and vitamin C supplements. Supplementary feeding of high-risk groups with CSM will also help to reduce the likelihood of anemia (CSM contains 18 g iron/100 g). Iron/folic acid. Routine iron/folate supplements should be provided to all pregnant and lactating women through antenatal and postnatal clinics. Female health workers should be employed to seek out pregnant and lactating women and encourage their participation in these programs. Vitamin C. Fortification of foods with vitamin C is problematic because vitamin C is unstable. Further study is needed on the appropriate vehicle for fortification. The best solution is to provide a variety of fresh foods either by including them in the general ration or by promoting access to local markets. In addition, local cultivation of vitamin C-containing foods should be encouraged. Patients with clinical scurvy should be treated with 250 mg of oral vitamin C two times daily for 3 weeks. Niacin. Maize-eating populations are at greatest risk for niacin deficiency, which causes pellagra. Recent studies of pellagra outbreaks among refugee populations found groundnut consumption, garden ownership, and home maize milling (as an indicator of higher socioeconomic status) to be protective factors. Niacin-fortified flour should be included in the general ration. The process of fortifying maize flour with niacin is simple and relatively inexpensive. Clinical cases of pellagra can be treated with nicotinamide. The recommended treatment schedule is 100 mg three times daily for 3 weeks. The total daily dose of nicotinamide should not exceed 600 mg. Where the diet is deficient in niacin, vitamin B complex tablets can be used to prevent pellagra. Iodine. If the general ration is naturally deficient of iodine, fortification of items such as salt or monosodium glutamate should be considered. References
Selected Reading Brown RE, Berry A. Prevention of malnutrition and supplementary feeding programs. In: Sandler RH, Jones TC, eds. Medical care of refugees. New York: Oxford University Press 1987:124. CDC. Outbreak of pellagra among Mozambican refugees Malawi, 1990. MMWR 1991;40:209-13. Desenclos JC, Berry AM, Padt R, Farah B, Segala C, Nabil AM. Epidemiological patterns of scurvy among Ethiopian refugees. Bull WHO 1989;67:309-16. Nieburg P, Waldman RJ, Leavell R, Sommer A, DeMaeyer EM. Vitamin A supplementation for refugees and famine victims. Bull WHO 1988;66:689-97. Peel S. Nutritional aspects of refugee assistance. In: Allegra DT, Nieburg P, Grabe M, eds. Emergency refugee health care--a chronicle of the Khmer refugee-assistance operation 1979-1980. Atlanta: CDC, 1983:121-7. Peel S. Selective feeding procedures. Oxfam Working Paper no.1. Oxford, 1979. Seaman J, Rivers J. Strategies for the distribution of relief food. J. R. Statist. Soc. 1988;151:464-72. United Nations Administrative Committee for Coordination, Subcommittee on Nutrition, and the International Nutrition Planners Forum. Nutrition in times of disaster. Presented as a report of an International Conference; September 27-30, 1988; Geneva, Switzerland. UNHCR/WFP. Guidelines for calculation food rations for refugees. Geneva/Rome. August 1991. Wallstam E, Nieburg P, Eie E, Lendorff A. Donated foods and their use in refugee-assistance operations. In: Allegra DT, Nieburg P, Grabe M, eds. Emergency refugee health care--a chronicle of the Khmer refugee-assistance operation 1979-1980. Atlanta: CDC, 1983:129-33. Yip R, Gove S, Farah BH, Mursal HM. Rapid assessment of hematological status of refugees in Somalia: the potential value of hemoglobin distribution curves in assessing iron nutrition status. Presented at the APHA annual meeting, October 20, 1987.
Vaccine-Preventable Diseases
OverviewOnly measles immunization should be part of the initial emergency relief effort; however, a complete EPI should be planned as an integral part of an ongoing long-term health program. Diphtheria, tetanus toxoids (TT) and pertussis vaccine (DTP), oral polio vaccine (OPV), and bacille Calmette-Guerin (BCG) vaccinations are recommended. None should not be undertaken, however, unless the following criteria are met: the population is expected to remain stable for at least 3 months; the operational capacity to administer vaccine is adequate, and the program can be integrated into the national immunization program within a reasonable length of time. It is essential that adequate immunization records be kept. At the very minimum, personal immunization cards (i.e., "Road to Health" cards) should be issued. In addition, a central register of all immunizations is desirable. MeaslesPriority. Measles vaccination campaigns should be assigned the highest priority early in emergency situations. Measles immunization programs should begin as soon as the necessary personnel, vaccine, cold chain equipment, and other supplies are available. Measles immunization should not be delayed until other vaccines become available or until cases of measles have been reported. In refugee populations fleeing from countries with high immunization coverage rates, measles immunization should still be accorded high priority. Studies of urban populations (e.g., Kinshasa, Zaire) and densely populated refugee camps (e.g., camps in Malawi) have shown that large outbreaks of measles may still occur even if vaccine coverage rates exceed 80%. For example, in a camp of 50,000 refugees, approximately 10,000 would be children less than 5 years of age. If the vaccine coverage rate was 80% and vaccine efficacy was 90%, approximately 2,800 children in this camp would still be susceptible to measles. In addition, certain countries achieved high coverage in the 12 to 23 month age group, leaving large numbers of older children unprotected. Program management. Responsibilities for each aspect of the immunization program need to be explicitly assigned to agencies and persons by the coordination agency. The national EPI should be involved from the outset of the emergency. National guidelines regarding immunization should be applied in refugee settings. A pre-immunization count should be conducted to estimate the number of children eligible for vaccination. This should not be allowed, however, to delay the start of the vaccination program. Choice of vaccine. The standard Schwarz vaccine is recommended. The use of medium or high titer Edmonston-Zagreb (E-Z) vaccine is not yet recommended for refugee populations, since there are still concerns about its safety. Target population. During the emergency phase, defined as that time during which the CMR is higher than 1/10,000/day, all children ages 6 months-5 years should be vaccinated upon arrival at the camp. In long-term refugee health programs, vaccination should be targeted at all children ages 9 months-5 years, except during outbreaks when the lower age limit should again be dropped to 6 months. Any child who has been vaccinated between the ages of 6 and 9 months should be revaccinated as soon as possible after reaching 9 months of age, or 1 month later if the child was 8 months old at first vaccination. If there is insufficient vaccine available to immunize all susceptible children, the immunization program should be targeted at the following high-risk groups, in order of priority:
Older children, adolescents, and adults may also need to be immunized if surveillance data show that these groups are being affected during an outbreak. Undernutrition is not a contraindication for measles vaccination! Undernutrition should be considered a strong indication for vaccination. Similarly, fever, respiratory tract infection, and diarrhea are not contraindications for measles vaccination. Unimmunized persons who are infected with HIV should receive the vaccine. Measles vaccine should also be administered in the presence of active TB (1). Outbreak control. Measles immunization programs should not be stopped or postponed because of the presence of measles in the camp or settlement. On the contrary, immunization efforts should be accelerated. Among persons who have already been exposed to the measles virus, measles vaccine may provide some protection or modify the clinical severity of the disease, if administered within 3 days of exposure. Isolation of patients with measles is not indicated in an emergency camp setting. Case management. All children who develop clinical measles in refugee camps should have their nutritional status monitored and be enrolled in a feeding program if indicated. Children with measles complications should be administered standard treatment, e.g., ORT for diarrhea and antibiotics for acute lower respiratory infection (ALRI). If they have not received vitamin A during the previous month, all children with clinical measles should receive 200,000 IU vitamin A orally. Children less than 12 months of age should receive 100,000 IU. This should be repeated every 3 months as part of the routine vitamin A supplementation schedule. Children with complicated measles (pneumonia, otitis, croup, diarrhea with moderate or severe dehydration, or neurological problems) should receive a second dose of vitamin A on day 2.
If any eye symptoms of vitamin A deficiency are observed (xerosis, Bitot's
spots, keratomalacia, or corneal ulceration), the following treatment
schedule should be followed: Diphtheria-tetanus-pertussisOnce a comprehensive EPI has been established, all children ages 6 weeks-5 years should receive three doses of DTP, 4-8 weeks apart. PoliomyelitisOne dose of OPV should be administered at birth, followed by three doses 4-8 weeks apart to all children 6 weeks-5 years of age. TuberculosisBCG vaccination should be offered as part of the comprehensive EPI, rather than as a separate TB program. One dose of BCG is administered subcutaneously at birth. Recommendations for TB control are presented in a separate section. Neonatal tetanusAll women between the ages of 15-44 years should receive a full schedule of TT vaccination. Vaccination should commence at a younger age if girls less than 15 years of age commonly bear children in the refugee community. TT vaccination should be included as part of a standard antenatal care program. Female health workers should be employed to educate women about the need for the TT vaccination and to refer pregnant women to the antenatal care clinic. Although WHO recommends a 5-dose schedule for TT vaccination (see "WHO Tetanus Toxoid Vaccination Schedule"), the number of doses of TT administered varies from country to country. The schedule in refugee camps should be consistent with host country national policies. Meningococcal meningitisSurveillance. In areas where epidemics of meningococcal meningitis are known to occur, as in Africa's "meningitis belt," surveillance for meningitis should be a routine part of a HIS. Such surveillance requires a standard case definition, the identification (in advance) of laboratory facilities and a source of supplies (e.g., spinal needles, antiseptics, test tubes), and a clearly established reporting network. Outbreak identification and control. If an outbreak of meningococcal meningitis is suspected, early priority should be given to the determination of etiology and serogroup. This may be accomplished through the use of latex agglutination tests. It is also important to determine antibiotic resistance patterns. Cerebral spinal fluid (CSF) or petechial washings should be placed in suitable transport media and kept at 37 C during transport to a local or regional laboratory with the capacity to perform the needed analysis. If transport media are unavailable, CSF specimens should be placed in a test tube and transported at body temperature as soon as possible. After an outbreak has been confirmed, a presumptive diagnosis of meningococcal meningitis among persons with suggestive symptoms and signs can be made by visual inspection of CSF from lumbar punctures; CSF will appear cloudy in probable cases. Clinical characteristics include fever, severe headache, neck stiffness, vomiting, and photophobia. Endemic rates of meningococcal disease vary by geographic area, season, and age; thus it is not possible to define a rate that can be applied universally to identify an epidemic disease. In one study, an average incidence rate of disease that exceeded 15 cases/100,000/week for a period of 2 consecutive weeks was predictive of an epidemic (defined as greater than 100 cases/100,000). Since this threshold may only be valid for populations greater than 100,000 and because the population in a refugee camp may be unknown, a doubling of the baseline number of cases from 1 week to the next over a period of 3 weeks may be used as a rough indicator of a meningitis outbreak. Vaccination. Vaccination of refugees against meningococcal meningitis during non-epidemic periods is generally not considered to be an effective measure because of the short duration of protection in young children. If there are compelling reasons to believe that the refugee population is at high risk for an epidemic, preventive vaccination before the meningitis season may be warranted. In the event of an outbreak, vaccination should be considered if the following criteria are met:
If it is logistically feasible, the household contacts of identified cases should be checked for vaccination status and immunized if necessary. It may be simpler to organize a mass immunization program. Because cases of meningococcal meningitis are likely to cluster geographically within a refugee camp, it may be most efficient to focus the vaccination campaign on the affected area(s) first. Although the target group for immunization should be determined from the epidemiology of the specific outbreak, vaccination of children and young adults between the ages of 1-25 years will generally cover the at-risk population. Chemoprophylaxis. Mass chemoprophylaxis is ineffective for control of epidemic meningococcal disease and is to be discouraged in a refugee setting. If chemoprophylaxis is to be instituted, the following guidelines should be implemented:
Rifampicin should not be administered to pregnant women. Patients should be warned that the drug will temporarily turn the urine and saliva orange. Ceftriaxone and ciprofloxacin may be used as alternatives to rifampicin. These drugs, like rifampicin, are expensive and are generally not considered appropriate in a refugee setting. Because of widespread resistance, sulfonamides should not be used unless susceptibility tests show the organism to be sensitive. Widespread use of rifampicin may encourage drug resistance and could cause iatrogenic morbidity due to adverse drug reactions. Treatment. IV-administered penicillin, which requires relatively intensive nursing care and medical equipment, is the treatment of choice for meningococcal disease in developed countries. However, in areas where such intensive care is not possible, a single intramuscular (IM) dose of long-acting chloramphenicol in oil suspension (Tifomycin) upon admission has been demonstrated to be effective. The dosage should be adjusted for age as follows:
In about 25% of cases, a second dose of chloramphenicol will be needed. Patients should be admitted as inpatients and monitored closely to determine whether the additional dose is required. The efficacy of this regimen of one or two doses of IM chloramphenicol has been proven in studies in both Europe and Africa. Febrile seizures are common in small children, and acetaminophen (paracetamol) in either oral suspension or rectal suppositories should be administered to patients upon admission. Typhoid and choleraVaccination for typhoid or cholera is not recommended in refugee situations. The resources required for such a campaign are better spent on improving sanitation conditions (see "Diarrheal Diseases"). Selected Reading CDC monograph. Allegra DT, Nieburg P, Eriksen H, Thousig O, Grabe M. Measles Outbreak, Khao I-Dang Refugee Camp, Thailand. In: Allegra DT, Nieburg P, Brabe M, eds. Emergency refugee health-care--a chronicle of experience in the Khmer assistance operation 1979- 1980. Atlanta, GA:1983;49-55. Moore PS, Toole MJ, Nieburg P, Waldman RJ, Broome CV. Surveillance and control of meningococcal meningitis epidemics in refugee populations. Bull WHO 1990;68:587-96. CDC monograph. Preblud SR, Horan JM, Davis CE. Meningococcal disease among Khmer refugees in Thailand. In: Allegra DT, Nieburg P, Brabe M, eds. Emergency refugee health-care -- a chronicle of experience in the Khmer assistance operation 1979-1980. Atlanta GA: 1983;65-9. CDC monograph. Preblud SR, Nieburg P, Allegra DT. Vaccination programs for refugees. In: Allegra DT, Nieburg P, Brabe M, eds. Emergency refugee health-care -- a chronicle of experience in the Khmer assistance operation 1979-1980. Atlanta GA: 1983;135-40. CDC monograph. Toole MJ, Foster S. Famines. In: Gregg MB, ed. The public health consequences of disasters 1989. Atlanta GA: 1989;85. United Nations Children's Fund (UNICEF). Assisting in emergencies: a resource handbook for UNICEF field staff. New York: United Nations Children's Fund, 1986:269-77.
Diarrheal DiseasesThe critical elements of a diarrheal disease control program in a refugee camp are: a) prevention of morbidity, b) prevention of mortality through appropriate case management, c) surveillance for morbidity and mortality attributed to diarrheal diseases, and d) preparedness for outbreaks of severe diarrheal diseases (e.g., cholera and dysentery). The objectives of a camp diarrheal diseases control program should include the following:
PreventionEfforts aimed at reducing the incidence of diarrheal diseases and other enterically transmitted diseases should focus primarily on the provision of adequate quantities of clean water, improvements in camp sanitation, promotion of breast-feeding, and personal hygiene education. The following recommendations relating to water and sanitation are largely based on the UNHCR Handbook for Emergencies (1) and Environmental Health Engineering in the Tropics (2). Water. In general, the supply of adequate quantities of water to refugees in a camp setting has greater overall impact on health than a supply of small quantities of microbially pure water. The provision of adequate quantities of water is particularly effective in the prevention of bacillary dysentery. Nevertheless, whenever possible, sources of clean water should be sought or disinfection systems established. An additional health benefit derived from the provision of ample supplies of water, at a convenient distance from the camp, is the decrease in the daily workload of women, upon whom the burden of water collection usually falls. Appropriate water sources should be identified before refugees arrive in an area. An adequate water supply is a crucial component of attempts to prevent disease and protect health and, as such, should be among the highest priorities for camp planners and administrators. Standards. WHO has set standards for the microbiological quality of water supplies. These are as follows:
The water quality should be tested before using a water source, at regular intervals thereafter, and during any outbreak of diarrheal disease in which the water source may be implicated. Sources. Whatever water source is chosen, it must be protected from contamination. Safety measures include:
Treatment. The selection of a water source should take into consideration the potential need for water treatment. Whether or not treatment is needed, the water should be tested routinely to ensure that it is of suitable quality. When surface water is used as a communal source, covered storage will allow suspended particles to settle on the bottom, improving the quality of the water. Longer standing times and higher temperatures will yield a greater improvement in water quality. Filtration and chlorination may require considerable effort and resources, but should be considered if the situation warrants. Although boiling is an effective means of removing water pathogens, it is not generally a practical solution in refugee camps where fuel supplies are limited. As a short-term measure during an emergency (e.g., a cholera outbreak, and when treatment of all water sources is not feasible), purification agents (such as chlorine) may be distributed to each household. In this way, water can be treated in household storage containers. However, a massive education effort is required and such measures usually cannot be maintained for longer than a few weeks. Water storage containers with narrow necks or covers that prevent people from introducing their hands into the container are likely to reduce further contamination of water once it is stored in the home. The use of separate containers to store water for drinking and water for washing is preferable. Supply. The chosen water supply should be adequate to meet the needs of the camp year-round. Seasonal variations in rainfall and in camp population should be taken into consideration when selecting a water source. The UNHCR recommends that a minimum quantity of 20 L of water/person/day be provided. Health clinics, feeding centers, and hospitals require 40-60 L/patient/day. Ideally, no individual dwelling should be located greater than 150 m from a water source. At any greater distance, the use of water for hygiene is greatly diminished. Sanitation. Camp sanitation plans should be drawn up before refugees arrive. Because of the crucial role it plays in disease prevention, sanitation should be an early priority for camp planners. Community attitudes and cultural practices regarding sanitation and disposal of excreta are vital to the success of a sanitation project and should be taken into careful consideration. All efforts should be made to separate garbage and human waste from water and food supplies. Excreta should be contained within a specific area. Defecation fields may be used as a short-term measure until a more appropriate sanitation system can be implemented. This is particularly suitable in hot, dry climates. The design and installation of latrines should also take into consideration the attitudes and practices of the refugee population. Latrines should be located so as to remove the possibility of contamination of the water source. Latrines that are poorly maintained will not be used. For this reason, personal or family latrines are the best solution. However, limitations on building supplies, money, and space may make this impossible. If communal latrines are to be used, no more than 20 people should share one latrine and responsibility for maintaining cleanliness should be clearly assigned. Breast-feeding. Breast-feeding is an effective measure for preventing diarrheal illness among infants. Exclusive breast-feeding for the first 4-6 months of a baby's life, and continued breast-feeding until the child is 2 years of age, should be encouraged through educational campaigns targeted at pregnant and lactating women. Distribution of milk products should be restricted, and feeding bottles should never be distributed within a camp (see "Nutrition"). Personal hygiene. Community health education should reinforce the importance of handwashing with soap and of general domestic and personal hygiene, in particular safe food-handling practices. Soap should be made readily available by relief agencies. Case managementAssessment (see "Patient Assessment"). An adequate history should be taken from the patient or the patient's family. The duration of illness; quantity, frequency, and consistency of stool; presence or absence of blood in the stool; frequency of vomiting; and the presence of fever or convulsions should be assessed. Assessment of dehydration and fluid deficit through careful physical examination should receive particular attention. Fever, rapid breathing, and hypovolemic shock may accompany severe dehydration. Careful monitoring of the patient's weight and the signs of dehydration throughout the course of therapy will help assess the adequacy of rehydration. Adults with acute, dehydrating diarrhea should be carefully assessed by a physician to rule out cholera. Management of patients. In the camp setting, all patients with diarrhea should be encouraged to report to a clinic or health post for assessment, advice on feeding, fluid intake, and diarrhea prevention. The treatment of dehydration should always be initiated in the clinic. Ideally, a central clinic should be supplemented with several small ORT centers in the camp, staffed by trained community health workers. Prevention of dehydration. Case management should focus on the prevention of dehydration under two sets of circumstances: a) when a patient with diarrhea shows no signs of dehydration, b) when a patient has already been treated for dehydration in the ORT corner and is being released from medical care. Management of patients in these situations includes the following. ORS. Mothers should be shown how to mix and give ORS and initially be given a 2-day supply. The amount to be given at home is as follows.
Increased fluids. Patients should be instructed to increase their normal intake of fluids. Any locally available fluids known to prevent dehydration, especially those that can be prepared in the home (e.g., cereal-based gruels, soup, and rice water), should be encouraged. Soft drinks are not recommended because of their high osmolality.
Continued feeding. Infants who are breast-fed should continue to receive
breast milk. If an infant is receiving milk formula in a feeding center, the
milk should be diluted with an equal volume of clean water until the diarrhea
stops.
Monitor condition. The mother should be advised to return to the clinic with the child if he/she continues to pass many stools, is very thirsty, has sunken eyes, has a fever, or does not generally seem to be getting better. Management of the dehydrated patientEvery health center in a refugee camp should have an area allocated for supervised oral rehydration (see "Guidelines for Rehydration Therapy"). Staff assigned to this activity need to be well-trained in the assessment and treatment of the dehydrated patient. Individual patients should be monitored to determine whether the recommended doses are adequate for their needs or whether rehydration proceeds faster than is expected. For babies who are unable to drink but are not in shock, a nasogastric tube can be used to administer ORS solution at the rate of 15 mL/kg body weight/hour. For infants in shock, a nasogastric tube should be used only if IV equipment and fluids are not available. Reassessment. The patient's hydration status should be reassessed after 3-4 hours, and treatment continued according to the degree of dehydration at that time. Note: If the child is still dehydrated, rehydration should continue in the center. The mother should offer the child small amounts of food. If the child is less than 12 months of age, the mother should be advised to continue breast-feeding. If the child is not being breast-fed, 100-200 mL of clean, plain water should be given before continuing the ORS. Older children and adults should consume plain water as often as they wish throughout the course of rehydration with ORS solution. Nutritional maintenance. Infants should resume feeding as outlined above. For children greater than 4-6 months old and adults, feeding should begin as soon as the appetite returns. Energy-rich, easily digestible foods will help maintain their nutritional status. There is no reason to delay feeding until the diarrhea stops and there is no justification for "resting" the bowel through fasting. Note: Children enrolled in SFPs or TFPs who develop diarrhea with dehydration should be fed HEM diluted with ORS in a ratio of 1:1, alternating with plain ORS. The overall volume of fluid should be calculated according to the child's weight and degree of dehydration. Use of chemotherapy. Antimicrobial drugs are contraindicated for the routine treatment of uncomplicated, watery diarrhea. Specific indications for their use include:
For specific recommendations see "Cholera" and "Dysentery". Anti-diarrheal agents are contraindicated for the treatment of diarrheal disease. Stimulants, steroids, and purgatives are not indicated for treatment of diarrheal disease and may produce adverse effects.
Surveillance for Diarrheal DiseasesAll health facilities that serve the refugee population should maintain case records of diarrheal diseases as part of the routine HIS. Records should include the degree of dehydration at the time of presentation. Case definitions should be standardized. Dysentery cases should be recorded as a separate category. Any increase in the number or severity of cases, change in the type of diarrhea, rise in diarrhea-specific mortality, or change in the demographic breakdown of the cases should be reported. A case definition for cholera should be established for the purpose of surveillance. Any suspected cholera cases should be reported immediately. Sample case definitions for cholera and dysentery are provided below.
CholeraIdentification of the pathogen by laboratory culture is necessary to confirm the presence of cholera. Initially, rectal swabs of patients with suspected cholera should be transported to the laboratory in Cary-Blair transport medium (see Collecting, Processing, Storing and Shipping Diagnostic Specimens in Refugee Health-Care Environments *). The laboratory should determine the antibiotic sensitivity of the cultured strain. Once an outbreak is confirmed, it is not necessary to culture every case. Additionally, it is not necessary to wait until an outbreak has been confirmed to begin treatment and preventive measures. * Available from IHPO, CDC, 1600 Clifton Road, MS F-03, Atlanta, GA 30333, 404-639-0308. Epidemics In the event of an outbreak of cholera, early case-finding will allow for rapid initiation of treatment. Aggressive case-finding by trained community health workers should be coupled with community education to prevent panic and to promote good domestic hygiene. Treatment centers should be easily accessible. Most patients can be treated with ORS alone in the local clinic and still achieve a CFR less than 1%. If the attack rate for cholera is high, it may be necessary to establish temporary cholera wards to handle the patient load. Health centers should be adequately stocked with ORS, IV fluids, and appropriate antibiotics. Health workers must be trained in the management of cholera. Surveillance should be intensified and should change from passive to active case-finding. The number of new cholera cases and deaths should be reported daily, along with other relevant information (e.g., age, sex, location in camp, length of stay in camp). Treatment The goal of cholera treatment is to maintain the CFR at less than 1%. Rehydration therapy Rehydration needs to be aggressive. However, careful supervision is necessary to prevent fluid overload, especially when children are rehydrated with IV fluids. Most cases of cholera can be treated through the administration of ORS solution (see "Patient Assessment" and "Guidelines for Rehydration Therapy". Persons with severe disease may require IV fluid, which should be administered following the guidelines outlined in "Diarrheal Diseases". Antibiotics Antibiotics reduce the volume and duration of diarrhea in cholera patients. Antibiotics should be administered orally. Doxycycline should be used when available in a single dose of 300 mg for adults and 6 mg/kg/day for children less than 15 years of age. Tetracycline should be reserved for severely dehydrated persons, who are the most efficient transmitters because of their greater fecal losses. Tetracycline should be administered according to the following schedule.
Chloramphenicol can be used as an alternative to tetracycline; the dosage is the same. When tetracycline and chloramphenicol resistance is present, furazolidone, erythromycin, or trimethoprim-sulfamethoxazole (TMP-SMX) may be used. Epidemiologic investigation Epidemiologic studies to determine the extent of the outbreak and the primary modes of transmission should be conducted so that specific control measures can be applied. The CFR should be monitored closely to evaluate the quality of treatment. Case-control studies may be undertaken to identify risk factors for infection. Environmental sampling, examination of food, and the use of Moore swabs for sewage sampling may be useful to confirm the results of epidemiologic studies and define modes of transmission. Control and prevention Health education. The community should be kept informed as to the extent and severity of the outbreak, as well as educated on the ease and effectiveness of treatment. Emphasis should be placed on the benefits of prompt reporting and early treatment. The community should be advised about suspected vehicles of transmission. The need for good sanitation, personal hygiene, and food safety should be stressed. Health workers involved in treating cholera patients need to observe strict personal hygiene, by washing their hands with soap after examining each patient. Smoking should be prohibited in cholera wards and clinics. Water supply. Any water supplies implicated through epidemiologic studies should be tested. Any contaminated water sources should be identified and access to those sources cut off. Alternative sources of safe drinking water should be identified and developed as a matter of urgency. Food safety. Community members should be informed of any food item that has been implicated as a possible vehicle of transmission. Health education messages regarding food preparation and storage should be disseminated. During an outbreak, feeding centers should be extremely vigilant in the preparation of meals because of the potential for mass infection. Food workers should have easy access to soap and water for handwashing. Food workers should always wash their hands after defecating, and any food worker who is experiencing diarrhea should be prohibited from working. Chemoprophylaxis. Mass chemoprophylaxis is not an effective cholera control measure and is not recommended. Although the WHO Guidelines for Cholera Control suggest that chemoprophylaxis may be justified for closed groups (such as refugee camps), CDC studies indicate that focusing on other preventive activities (i.e., providing an adequate water supply, improving camp sanitation, and providing adequate and prompt treatment) results in a more effective use of resources. If resources are adequate and transmission rates are high (greater than 15%), consideration should be given to providing a single dose of doxycycline to immediate family members of diagnosed patients. Vaccines. Currently available vaccines are not recommended for the control of cholera among refugee populations. The efficacy of these vaccines is low and the duration of protection provided is short. Vaccination campaigns divert funds and personnel from more important cholera control activities and give refugee and surrounding populations a false sense of security.
DysenteryWhen possible, patients presenting with signs and symptoms of dysentery should have stool specimens examined by microscopy to identify Entamoeba histolytica. Care should be taken to distinguish large white cells (a nonspecific indicator of dysentery) from trophozoites. Amebic dysentery tends to be misdiagnosed. ShigellosisIf a microscope is unavailable for diagnosis, or if definite trophozoites are not seen, persons with bloody diarrhea should be treated initially for shigellosis. Appropriate treatment with antimicrobial drugs decreases the severity and duration of dysentery caused by Shigella and reduces the duration of pathogen excretion. The selection of an antimicrobial treatment regimen is often complicated by the presence of multiresistant strains of Shigella. The choice of a first-line drug should be based on knowledge of local susceptibility patterns. If no clinical response occurs within 2 days, the antibiotic should be changed to another recommended for that particular strain of shigellosis. If no improvement occurs after an additional 2 days of treatment, the patient should be referred to a hospital or laboratory for stool microscopy. At this stage, a diagnosis of resistant shigellosis is still more likely than amebiasis. Drugs of choice. Treatment guidelines for shigellosis are listed below.
For strains resistant to these regimens, alternative treatment with nalidixic acid or tetracycline is indicated.
The fluoroquinolones (e.g., ciprofloxacin and ofloxacin) are highly effective for the treatment of shigellosis, but are expensive and have not yet been approved for treatment of children or pregnant or lactating women with shigellosis. Because multiresistant strains of Shigella have become widespread and because Shigella strains can rapidly acquire resistance in endemic and epidemic settings, it is advisable that periodic antibiotic susceptibility testing be performed by a reference laboratory in the region. Note: WHO does not recommend mass prophylaxis or prophylaxis of family members as a control measure for shigellosis. Amebiasis and giardiasisTreatment for amebiasis or giardiasis should not be considered unless microscopic examination of fresh feces shows amebic or Giardia trophozoites, or two different antibiotics given for shigellosis have not resulted in clinical improvement. Treatment guidelines for amebiasis are as follows:
Treatment guidelines for giardiasis are as follows:
References
Selected Reading World Health Organization. A manual for the treatment of acute diarrhoea. Geneva: Diarrhoeal Diseases Control Programme, 3rd. ed., 1990. CDC. Shigella dysenteriae Type 1 Guatemala, 1991. MMWR 1991;40:421,427-8. Keusch GT, Bennish ML. Shigellosis: recent progress, persisting problems and research issues. Pediatr Infect Dis J 1989;8:713-9. Smith M. Water and sanitation for disasters. Trop Doct 1991;21(suppl 1):30-7. World Health Organization. Guidelines for cholera control. 1991;80.4;Rev. 2.
MalariaKnowledge of the epidemiology of transmission, including local vectors, is essential to a malaria control effort. Information regarding the local epidemiology may be available from the MOH, WHO, and regional health authorities. In certain instances, a vector survey may need to be done. The national malaria control program or WHO staff are often able to conduct such surveys. Information on previous exposure can be obtained from the refugees themselves, or more detailed information on previous exposure to specific species can be obtained through international channels via WHO. Within a camp, the proportion of fever illness attributable to malaria at a particular time can be determined by obtaining thick and thin blood smears from a sample of consecutive clinic patients with a history of recent fever (e.g., 50 children less than 5 years of age). The malaria infection prevalence rate among these patients can then be compared with a control group that is free of the signs and symptoms of malaria. Laboratory examination will determine whether malaria illness is caused by Plasmodium falciparum or Plasmodium vivax. Control of TransmissionControl of malaria transmission may be achieved through a combination of the following strategies. Personal protection. The use of protective clothing, insecticide-impregnated bed nets, and insect repellents will help limit human exposure to malaria-infected mosquitoes. Residual insecticides. Periodic spraying of the inside surfaces of permanent dwellings may reduce transmission. The use of residual insecticides, however, may be toxic to those involved in spraying and can also be detrimental to the environment. Spraying can be expensive and time consuming. Careful consideration should be given to the technical aspects of spraying, local vector behavior and susceptibility, personnel training, safety, and community motivation before undertaking such a program. Source reduction. The elimination of breeding sites by draining or filling may reduce the density of vectors in the area. Knowledge of the local vectors is essential to ensure that source reduction efforts are effectively targeted. Ultra low-volume insecticide spraying. Adult mosquitoes may be killed through frequent fogging with nonresidual insecticides. Fogging is generally repeated on a daily basis. Gametocidal drug use. Gametocidal drugs (e.g., primaquine) are not generally recommended for use in refugee camps. Selection of control strategies will depend upon the local epidemiologic factors, availability of resources, and environmental and cultural factors. Case ManagementCase definition. Malaria infection is defined as the presence of malaria parasites in the peripheral blood smear. Malaria illness is defined as the presence of "malaria signs and symptoms" in the presence of malaria infection. The signs and symptoms of malaria typically include fever, chills, body aches, and headache. Diagnosis. If possible, a thick blood smear and Giemsa stain should be the basis for the diagnosis of malaria. These smears will also provide the basis for transmission surveillance in camps or geographic areas. If the patient load exceeds the capability of the laboratory to perform thick smears on all suspected cases, a system of microscopic diagnosis for a percentage of suspected cases should be established. When diagnoses are made by locally trained microscopists in small field laboratories, a randomly selected sample of both positive and negative slides should be sent to a reference laboratory for verification in order to maintain quality control. When laboratory facilities are not available, clinical symptoms (paroxysmal fever, chills, sweats, and headache) and signs (measured fever) are the best predictors of malaria infection. In situations in which year-round high malaria endemicity has been established, all episodes of fever illness can be assumed to be caused by Plasmodium falciparum. However, health workers should bear in mind other causes of fever, including pneumonia, ALRI, or meningitis. In areas where transmission is highly seasonal, surveys should be conducted each year at the beginning of the high transmission season. The presence of Plasmodium on blood smears does not prove that malaria is the cause of febrile illness, even in areas where malaria is highly prevalent. Other causes should be considered and ruled out. Treatment with chemotherapy. In areas without chloroquine resistance, the oral regimen of chloroquine usually employed in the treatment of uncomplicated attacks of malaria is as follows:
In areas where the likelihood of reinfection is low, consideration may be given to supplementation of chloroquine treatment with primaquine for persons infected with Plasmodium vivax.
Among populations in which severe glucose-6-phosphate dehydrogenase (G-6-PD) deficiency is common (notably among Asians), however, primaquine should not be administered for greater than 5 days. Administration of primaquine for longer periods may result in life-threatening hemolysis. Whenever possible, persons needing primaquine should first have a blood test for G-6-PD deficiency. When laboratory analysis is performed, the first dose of chloroquine should be administered when the blood smear is taken. The patient should be instructed to return the second day for the results of the smear. If the smear is positive, chemotherapy should be continued. If the smear is negative and the patient remains febrile, other causes of fever should be identified. If supervised therapy during a 3-day period is not possible, the first dose of chloroquine should be given under supervision and the additional doses may be given to the patient with appropriate instructions. Patients who remain symptomatic longer than 3 days into therapy should have a repeat thick smear examined. Alternative therapy should be instituted if the degree of parasitemia has not diminished markedly by this time. In areas with chloroquine resistance, treatment of patients may be the same as in areas of chloroquine-sensitive malaria; or may include an alternative first-line drug. Additional care in the follow-up of patients is required.
Fever control. Antipyretics (i.e., acetaminophen, paracetamol) and anticonvulsives are often necessary for the care of the patient with malaria. Children with high fevers should be frequently sponged with tepid water. Patients should increase their intake of fluids as the febrile illness will most likely be accompanied by mild dehydration. Patients with signs of moderate dehydration should be given ORS. ChemoprophylaxisDuring epidemics (seasons of high rates of transmission), malaria chemoprophylaxis should be considered for the following high-risk groups:
The decision to provide chemoprophylaxis to high-risk persons should be based upon the capabilities of the health-care system to accomplish the following:
Administration of chemoprophylaxis to high-risk groups can be logistically difficult and may be too great a strain on the capacities of the health-care system to be feasible. Expatriates working in an endemic area should be on weekly chloroquine (300 mg chloroquine base) during the entire period of exposure and for an additional 6 weeks after leaving the area. In areas where chloroquine resistance is documented, prophylaxis with mefloquine is recommended (250 mg weekly dose). Severe malariaSevere malaria is considered a medical emergency and demands prompt and specific medical care. Signs and symptoms of severe malaria include:
Signs of abnormal central nervous system (CNS) function, which may be present in cerebral malaria, include drowsiness, mental confusion, coma, and seizures. Management of severe malaria. The following guidelines for the management of severe malaria are based upon those prepared by the MOH in Malawi. Outpatient setting. If severe malaria is diagnosed in an outpatient setting, the patient should be referred for hospitalization. However, treatment should begin immediately and not be delayed until the patient has been transferred. If the patient can swallow, sulfadoxine-pyrimethamine (SP) tablets (500 mg-25 mg) should be administered orally in the following doses according to the patient's age.
If the patient vomits within 30 minutes, the dose should be repeated. If the patient cannot swallow or is vomiting repeatedly, an IM injection of quinine dihydrochloride (10 mg/kg) should be administered. This can be repeated every 4 hours for two additional doses, and every 8 hours thereafter if a long delay is anticipated for transport of the patient to a hospital. The patient's fever should be reduced by sponging with lukewarm water or by using paracetamol or aspirin. Patients should be given ORS. In a patient who cannot drink, administer 20 mL/kg ORS with one teaspoon of glucose powder via naso-gastric tube every 4 hours. If convulsions occur, administer 0.2 mL/kg paraldehyde by IM injection. If convulsions recur, repeat the treatment. If convulsions persist, give the patient a phenobarbitone 10-mg/kg IM injection. In a child with altered consciousness or repeated convulsions, the physician should perform a lumbar puncture if possible. If the CSF is cloudy, treatment for meningococcal meningitis is indicated and anti-malarial treatment should be discontinued. If a lumbar puncture cannot be performed, treatment for meningitis should be administered while continuing treatment for malaria. Inpatient setting. The following tests should be performed immediately upon admission: thick blood film, hemoglobin, blood glucose, and lumbar puncture. If hemoglobin is below 4 g/dL, blood grouping and cross-matching should be done. If the patient can swallow, give oral SP as described above. If the patient cannot swallow or has persistent vomiting, give IV-administered quinine as follows:
In the presence of signs of volume depletion, fluid (which includes dextrose) should be administered to maintain cardiac output and renal perfusion.
Hypoglycemia is a complicating factor in patients with cerebral malaria and a risk factor for fatal outcome. When possible, blood glucose levels should be monitored. Hypoglycemia should be suspected whenever there is a deterioration in clinical status, especially in the presence of new neurologic findings. Hypoglycemia can be treated presumptively with 50 mL of 50% IV dextrose. Blood transfusion is indicated when Hb less than 4 g/dL, or Hb less than 6 g/dL is detected and the patient has signs of heart failure (i.e., dyspnea, enlarging liver, gallop rhythm). The administration of steroids has an adverse effect on outcome in cerebral malaria. Therefore, steroids are no longer recommended. AnemiaMost anemias caused by malaria will reverse spontaneously after anti-malarial therapy. However, anemia may progress for several weeks after successful treatment of severe malaria and may require treatment. For some patients (especially children), blood transfusion may be lifesaving. Recent studies indicate that blood transfusion should be given for Hb less than 4 g/dL or Hb less than 6 g/dL in the presence of symptoms of respiratory distress. Because of the potential for HIV or hepatitis B transmission, blood transfusion should be reserved for medical emergencies for which no alternative treatment exists. Facilities for screening blood for HIV antibodies are rare in refugee camps. Whenever feasible, patients requiring transfusion should be transferred to hospitals where such facilities exist. The anemia of malaria is not associated with iron loss, and replacement is helpful only if a coexisting iron deficiency exists. Folic acid replacement may be helpful during the recovery period when rapid erythrocyte replacement occurs. Renal failureReplacement of fluid losses (sweat, vomit, and diarrhea) is recommended to prevent renal failure. If renal failure is suspected, strict monitoring of fluid intake and output is necessary. In the presence of oliguria, a fluid challenge followed by furosemide injection can help to differentiate acute renal failure from prerenal causes. If renal failure is demonstrated, fluid intake must be limited to daily replacement of insensible loss plus urine/vomitus volume in the previous 24 hours. Protein intake should be limited to less than 30 mg/day, and all drug doses should be adjusted for renal failure. Selected Reading Ministry of Health. Malawi guidelines for the management of malaria. Malawi, October 1991. CDC. Steketee RW, Campbell CC. Control of malaria among refugees and displaced persons. Atlanta, GA:1988 (unpublished).
TuberculosisThe TB control program should establish a policy covering areas of case definition, case-finding, treatment regimen, and the supervision of chemotherapy. This policy should be agreed upon and adhered to by all organizations and agencies providing health services to the refugees. During the emergency phase of a refugee relief operation, TB activities should be limited to the treatment of patients who present themselves to the health-care system and in whom tubercle bacilli have been demonstrated by sputum smear examination. Control of transmissionTarget population. Because of the limited resources available, efforts to control transmission of TB within a refugee settlement should focus on the primary sources of infection, i.e., those patients for whom microscopic analysis of sputum smears demonstrates the presence of acid-fast bacilli (AFB). (Specimens should be stained using the Ziehl-Neelsen method with the results graded quantitatively.) Case identification. Passive case-finding will be most efficient in the refugee setting. Patients with respiratory symptoms (chest pain, cough) of greater than 3 weeks' duration, hemoptysis of any duration, or significant weight loss should have a direct microscopic examination of their sputum for AFB. If the sputum smear is negative for AFB but pulmonary TB is still suspected, the patient should be given a 10-day course of antibiotics and then be re-examined after 2-4 weeks. Specific anti-TB chemotherapy should not begin unless the presence of AFB has been confirmed. Symptomatic family members of an identified patient should also have sputum specimens examined. Children who show signs and symptoms compatible with TB and who are either: a) a close contact of a patient with a confirmed case of TB, or b) tuberculin skin-test positive (in the absence of a BCG vaccination scar) should undergo a full course of anti-TB treatment if they do not respond to an appropriate regimen of alternative antibiotics. Case management. The selection of a first-line chemotherapy regimen should generally be consistent with the national policy set forth by the host country MOH. However, it should be recognized that the crowded conditions of a refugee camp may foster an abnormally high rate of transmission. Additionally, uncertainty exists regarding the duration of stay in the country of asylum, and it may be more difficult to maintain adherence to an extended therapy regimen. Short-course therapy (6 months) should be considered for use in a refugee camp even when the national policy prescribes a longer course of treatment, provided the additional expense is not prohibitive. Before enrolling refugees in a TB treatment program, consideration should be given to the stability of the populations and the capacity of the health-care program to supervise therapy and to follow-up patients who do not adhere to treatment. Administration of anti-TB drugs to persons in whom adherence is likely to be sporadic will foster increased drug resistance in that population. The following drugs are used for the treatment of TB with chemotherapy: isoniazid, rifampin, pyrazinamide, streptomycin, ethambutol, and thiacetazone. The selection of a particular treatment regimen must take into consideration the organism susceptibility, cost, and duration of therapy. The decision regarding implementation of a specific therapeutic regimen will generally be made by the UNHCR in consultation with the MOH of the host government. Case-holding. Whenever possible, chemotherapy should be observed by a health-care provider, especially during the first 2-3 months of treatment. Treatment efficacy should be assessed through a series of sputum smears. Patients participating in observed therapy who do not respond to treatment and whose sputum smears remain positive for AFB after 2 months should be reviewed by a physician and should begin a second-line treatment regimen. Enrolling TB patients in a SFP may improve adherence to the treatment regimen and acts as a point of contact for follow-up. The success of a TB control program depends on good management and close supervision. The responsibilities of staff assigned to the program need to be clearly defined, adequate records of patient progress should be maintained, and a system to follow-up patients who do not adhere to treatment should be established. The cooperation of the community is essential for success. A community education program should be established to help ensure adherence. PreventionPreventive chemotherapy for subclinical TB usually does not play a substantial role in TB control in a refugee camp. However, immediate family members of active TB patients should be examined for active TB and referred for treatment. This is particularly important for young children. BCG vaccination should be administered as part of the comprehensive immunization schedule and not as a separate TB control activity. BCG vaccination is contraindicated for persons with symptomatic HIV infection, but can be administered to asymptomatic persons. Selected Reading Rieder HL, Snider DE, Toole MJ, et al. Tuberculosis control in refugee settlements. Tubercle 1989;70:127-4. Davis CE, Allegra DT, Buhrer M. Tuberculosis control programs, Sakaeo and Khao I-Dang. In: Allegra DT, Nieburg P, Grabe M, eds. Emergency refugee health care -- a chronicle of the Khmer refugee-assistance operation 1979-1980. Atlanta GA: CDC;1983:61-4.
Epidemic InvestigationsAn epidemic is an unusually large or unexpected increase in the number of cases of a certain disease for a given place and time period. The general conditions of many refugee settlements (i.e., overcrowding, poor water and sanitation, inadequate rations) create an environment conducive to epidemics of infectious diseases. In the event of a suspected outbreak, an epidemiologic investigation should be conducted as quickly as possible. PurposeEpidemiologic investigations are conducted in order to:
PreparationsEach camp should have an established HIS with standardized reporting practices. This will allow for prompt recognition of and rapid response to an epidemic. An accurate assessment of available laboratory facilities is necessary in order to identify appropriate sites for microbiologic confirmation of an epidemic and to address deficiencies that may hamper an investigation. Appropriate specimen containers and transport media should be procured. Arrangements should be made to meet the need for additional technical support. A recognized administrative and reporting structure should be established, with a clear chain of command and delegation of responsibility. Lines of command should be well defined, and specific persons should be assigned responsibility for addressing the media and acting as liaisons to the camp leaders and the refugee population. Current maps showing settlements, water sources, transport routes, and health facilities should be made available to investigators. Conducting the investigationDetermining the existence of an epidemic. An established HIS will allow for prompt recognition and confirmation of an epidemic. The need for routine health surveillance in a refugee camp cannot be overstated. Even if such a system is firmly in place and implemented, reports of an epidemic may be the result of artifactual causes, i.e., changes in reporting practices, an increased interest in a particular disease, a change in diagnostic methods, the arrival of new health staff, or an increase in the number of health facilities. Confirming the diagnosis. The diagnosis of an epidemic disease should be confirmed using standard clinical or laboratory techniques. However, once the presence of an epidemic is established, it is not necessary to confirm the diagnosis for each person before treatment. Ongoing laboratory confirmation of a sample of cases is generally sufficient. Determining the number of cases. A workable case definition must be established in order to determine the scope of the outbreak. The sensitivity and specificity of the case definition depend upon:
A case-finding mechanism should be established. The dynamics of this system will depend upon the disease being investigated and the specific attributes of the camp involved. Case-finding will be facilitated if a cadre of refugee community health workers has been identified and trained. The presence of an active camp health committee will also promote effective case-finding. Time, place, and person. Certain information should be collected from each patient, or from their families, and recorded in a register. This should include:
Determining who is at risk. The data collected from patients should be used in an ongoing analysis to determine who is at greatest risk and to target specific interventions most effectively. Prepare a graph showing the number of cases per day. This "epidemic curve" will indicate the point at which the outbreak first occurred, the magnitude of the outbreak, the incubation period, and possible modes of transmission. Using a current map of the camp, mark the residence or section of the camp of each case as it is reported. This will allow investigators to identify clusters of patients and may help to pinpoint a common source of infection. A breakdown of cases by age, gender, length of stay in camp, vaccination status, if pertinent, and perhaps ethnic group will enable investigators to identify those groups or persons who are at highest risk for infection. Testing a hypothesis. As preliminary data are collected and analyzed, a hypothesis on the causative exposure should be developed and tested. A case-control study and analysis will help determine likely risk factors and sources of exposure. Laboratory analysis of environmental samples may be used to confirm a suspected source of infection. Preparing a report. Meetings should be held regularly with camp administrative officials, UNHCR and NGO representatives, local health officials, and refugee community leaders to discuss the evolution of the outbreak and to stress current control measures. In some cases, a written report may be necessary before any control and prevention efforts are undertaken. The report should include an estimate of the magnitude and health impact of the outbreak in numbers of projected cases and deaths. It should also include an estimation of the need for outside assistance and supplies. A written report will also provide a valuable record for use in future investigations. Moreover, the written report can serve as a useful teaching tool. Control and prevention As the epidemiologic investigation progresses, it is important that decision-makers be informed as to the findings so that appropriate control measures may be instituted. Continued disease surveillance will determine the effectiveness of control measures. Selected Reading CDC Monograph. Toole MJ, Foster S. Famines. In: Gregg MB, ed. The public health consequences of disasters 1989. Atlanta, GA: 1989.
POINT OF CONTACT FOR THIS DOCUMENT:To request a copy of this document or for questions concerning this document,
please contact the person or office listed below. If requesting a document,
please specify the complete name of the document as well as the address to
which you would like it mailed. Note that if a name is listed with the
address below, you may wish to contact this person via CDC WONDER/PC e-mail.
For single issue purchase 800-843-6356
Table 1TABLE 1. Refugees and asylum seekers by geographic region, July 1991 =============================================================================== Area of asylum Total refugee population ------------------------------------------------------------------------------- Africa 5,100,000 East Asia and the Pacific 1,400,000 Europe and North America 3,400,000 Latin America and the 1,100,000 Caribbean Middle East and Southwest Asia 17,100,000 ------------------------------------------------------------------------------- Source: UNHCR, 1992. =============================================================================== Table 2TABLE 2. Monthly crude mortality rates (CMRs) in selected refugee populations, with
baseline CMRs from countries of origin, 1978-1991
===================================================================================================
Host
Month/year (ref) country Country of origin Baseline CMR Refugee CMR
---------------------------------------------------------------------------------------------------
Jun-Dec 1978 (1) Bangladesh Burma (1.0) 6.3
Oct 1979 (1) Thailand Cambodia (2.5) 31.9
Aug 1980 (1) Somalia Ethiopia (2.0) 30.4
Jan-Mar 1985 (1) Sudan Ethiopia (2.0) 16.2
Sep 1985 (1) Sudan Chad (1.6) 24.0
Jan-Jun 1987 (1) Malawi Mozambique (1.5) 1.0
Sep 88-Aug 89 (9) Ethiopia Somalia (1.8) 3.8
Jul 1990 (9) Ethiopia Sudan (1.7) 6.9
Jun 1991* Ethiopia Somalia (1.8) 14.0
Apr 1991 (10) Turkey Iraq (0.7) 12.6
---------------------------------------------------------------------------------------------------
*Bhatia R, personal communication.
===================================================================================================
Table 3TABLE 3. Monthly crude mortality rates (CMRs) among selected internally displaced
populations, 1982-1990
=========================================================================================
Baseline CMR Displaced
Month/year (ref) Country (death/1,000) population CMR
-----------------------------------------------------------------------------------------
Nov 82-0ct 83 (1) Mozambique 1.4 8.0
Oct-Dec 1984 (1) Ethiopia (Korem) 2.0 60-90
Oct 84-Jan 85 (11) Ethiopia (Harbu) 2.0 147
Jul 1988 (1) Sudan 1.7 90
Jan-Dec 1990* Liberia 1.1 7.1
-----------------------------------------------------------------------------------------
*Holland MSF, unpublished data.
=========================================================================================
Table 4Table 4. Monthly crude mortality rates (CMRs) among selected nondisplaced
famine -affected populations, 1984-1985
==============================================================================================
Baseline CMR Famine-affected
Month/year (ref) Country/region (deaths/1000) CMR
----------------------------------------------------------------------------------------------
Oct 84-Jan 85 (12) Ethiopia(Shoa) 2.0 8.8
Jan-Dec 85 (11) Sudan (Darfur) 1.7 3.3
Jan-Dec 85 (11) Sudan (N.Kordofan) 1.7 7.8
==============================================================================================
Table 5TABLE 5. Prevalence of acute undernutrition (<80% median weight-for-height)
among children <5 years of age in refugee populations
===================================================================================================
Host Country Prevalence (%)
Dates (ref) country of Origin Population acute undernutrition
---------------------------------------------------------------------------------------------------
1979 (15) Thailand Kampuchea 31,900 10.0-18.0
1980 (16) Somalia Ethiopia 700,000 21.7-28.4
1984-85 (17) Pakistan Afghanistan 2,500,000 2.3-3.5
1988 (18) Malawi Mozambique 400,000 2.1-6.1
1988-89 (19) Ethiopia Somalia 400,000 12.9-29.5
1990 (20) Guinea Liberia 400,000 5.3
1990* Ethiopia Sudan 25,000 45.0
1991+ Kenya Somalia 50,000 29.0
1991 (10) Iraq/Turkey Iraq 400,000 4.1
Border
---------------------------------------------------------------------------------------------------
*UNHCR-Kenya. MacAskill J, December 1991.
+CDC. Toole M, trip report, July 1990.
===================================================================================================
Table 6TABLE 6. Prevalence of acute undernutrition (<80% of median weight-for-height)
among children <5 years of age in famine-affected populations
===================================================================================================
Population Prevalence (%)
Dates (ref) Affected region affected acute undernutrition
---------------------------------------------------------------------------------------------------
1983 (23) Mauritania 1,600,000 11.6-22.5%
1984-85 (24) Niger (5,400,000)* 9.8-13.7%
1985 (25) Burkina Faso (7,200,000)* 5.7-10.6%
1985 (26) Chad (4,500,000)* 25.8-56.0%
1985 (27) Somalia unspecified 11.2-23.5%
1990 (28) Haiti unspecified 4.2%
---------------------------------------------------------------------------------------------------
*Population given is national population.
===================================================================================================
Table 7TABLE 7. Prevalence of acute undernutrition (<80% of median weight-for-height)
among children <5 years of age in internally displaced populations
===================================================================================================
Population Prevalence
Date (ref) Country/region affected acute undernutrition
---------------------------------------------------------------------------------------------------
1983 (29) Mozambique 12-28%
1985 (11) Ethiopia (Korem) 800,000 70%
1988 (30) Sudan (Khartoum) 750,000 23%
1988 (30) Sudan (S. Darfur) 80,000 36%
1990* Liberia (Monrovia) 500,000 35%
---------------------------------------------------------------------------------------------------
*Holland MSF, unpublished data.
===================================================================================================
Table 8TABLE 8. Micronutrient deficiency disease outbreaks in refugee camps, 1984-1991
=========================================================================================
Disease Year (ref) Location Prevalence (%)
-----------------------------------------------------------------------------------------
Scurvy 1984 (32) Sudan 22.0
1985 (32) Somalia 6.9-44.0
1989 (19) Ethiopia 1.0-2.0
1991 (33) Sudan NA
Xerophthalmia 1985 (34) Sudan NA
Beriberi 1985 (1) Thailand NA
Pellagra 1989 (35) Malawi (11 camps) 0.5
1990 (35) Malawi (11 camps) 6.3
Iron Deficiency
Anemia 1990* Syria, Jordan, 54.5-73.9
West Bank & Gaza (children)
12.5-62.5
(women)
1990+ Ethiopia 10.0-13.0
-----------------------------------------------------------------------------------------
*CDC, unpublished data.
+SCF, unpublished data.
=========================================================================================
Figure 1Countries With Major Refugee Populations
Figure 2Countries With Major Internally Displaced Populations
Figure 3Crude Mortality Rates For Persons In Refugee Camps
Figure 4Major Causes Of Death In Refugee Populations
Figure 5Major Causes Of Death In All Ages
Figure 6Major Reported Causes Of Death In Children
Figure 7Mortality Rates In 41 Refugee Camp Populations
Figure 8PEM Prevalence In Children
Figure 9Measles Mortality In Wad Kowli Refugee Camp
Figure 10Proportion Of Outpatients With Diarrhea
Figure 11Cholera Cases And Deaths In Gannet
Figure 12Cholera Cases Reported in Nyamithutu Camp
Figure 13Hepatitis Cases Reported Among All Age Groups
Approved for public release; Distribution is unlimited. The listing of any non-Federal product in this CD is not an endorsement of the product itself, but simply an acknowledgement of the source. Operational Medicine 2001 Health Care in Military Settings
This web version is provided by The Brookside Associates Medical Education Division. It contains original contents from the official US Navy NAVMED P-5139, but has been reformatted for web access and includes advertising and links that were not present in the original version. This web version has not been approved by the Department of the Navy or the Department of Defense. The presence of any advertising on these pages does not constitute an endorsement of that product or service by either the US Department of Defense or the Brookside Associates. The Brookside Associates is a private organization, not affiliated with the United States Department of Defense. |