3rd Trimester Bleeding
Bloody
show · Cervicitis
and Trauma · Placental
abruption · Placenta
Previa · Clinical
approach · Shock · Blood
Transfusion · Transfusion without a Blood Bank
The third trimester has special clinical significance,
encompassing problems that are quite serious and those that are normal
or expected. It is important to be able to distinguish between them.
Bloody show
As the cervix thins (effaces) and begins to dilate in
preparation for labor, the patient may notice the passage of some bloody
mucous. This is a normal event during the days leading up to the onset
of labor at term. If this is the only symptom, the patient can be
reassured of its normalcy. If the patient is also having significant
contractions, she should be evaluated for the possible onset of labor.
If this bloody mucous show appears prior to full term, then it may
signal the imminent onset of preterm labor. These patients are evaluated
for possible pre-term labor.
Read
more about the evaluation and management of pre-term labor.
Bleeding that is more than bloody mucous (bright red, no
mucous, passage of clots) requires further evaluation.
Cervicitis and cervical
trauma
During pregnancy, the cervix becomes softer, more fragile, and
more vulnerable to the effects of trauma and microbes.
Cervical ectropion, in which the soft, mucous-producing endocervical
mucosa grows out onto the exocervix is common among pregnant women. This
friable endocervical epithelium bleeds easily when touched. This
situation can lead to spotting after intercourse, a vaginal examination,
or placement of a vaginal speculum.
Cervical ectropion also can lead to cervicitis. The normal squamous
cell cervical epithelium is relatively resistant to bacterial attack.
The endocervical mucosa is less resistant. If infected, the cervical
ectropion is even more likely to bleed if touched.
These changes are usually easily seen during a vaginal speculum
exam.
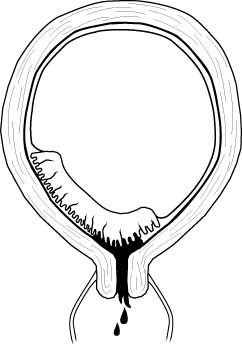
Placental abruption
Placental abruption is also known as a premature separation of
the placenta. All placentas normally detach from the uterus shortly after
delivery of the baby. If any portion of the placenta detaches prior to birth of
the baby, this is called a placental abruption. Placental abruption occurs in
about 1% of all pregnancies.
A placental abruption may be partial or complete.
-
A complete abruption is a disastrous event. The fetus will die within
15-20 minutes. The mother will die soon afterward, from either blood loss or the
coagulation disorder which often occurs. Women with complete placental abruptions are
generally desperately ill with severe abdominal pain, shock, hemorrhage, a rigid and
unrelaxing uterus.
-
Partial placental abruptions may range from insignificant to the
striking abnormalities seen in complete abruptions.
Clinically, an abruption presents after 20
weeks gestation with abdominal cramping, uterine tenderness, contractions, and usually
some vaginal bleeding. Occasionally, the blood loss is trapped inside the
uterus. These cases are called "concealed abruptions."
A number of factors are
associated with an increased risk of placental abruption, among them:
-
Prior placental
abruption (roughly doubles the risk of abruption in a
subsequent pregnancy)
-
Abdominal
trauma, including motor vehicle accidents
-
High maternal
parity
-
Low
socio-economic status
-
Poor nutrition
|
|
Abruptions are often
diagnosed clinically, based on the symptoms of bright red vaginal
bleeding, frequent contractions and uterine tenderness. There are no
laboratory findings that are specific for placental abruption. In mild
cases, laboratory tests are usually normal. In more advanced cases, the Hgb and Hct go down, as do the platelets and fibrinogen (due to the massive bleeding and consumption of
coagulation factors) while fibrin split products go up. Large numbers of
fetal RBCs may be identified in the maternal blood.
In the case of large
abruptions, ultrasound may identify a retroplacental blood clot. In
milder cases, ultrasound scans are frequently normal.
Mild abruptions may resolve with bedrest and observation, but
the moderate to severe abruptions generally result in rapid labor and delivery of the
baby. If fetal distress is present (and it sometime is), rapid cesarean section may be
needed.
Because so many coagulation factors are
consumed with the internal hemorrhage, coagulopathy is common. This means that even after
delivery, the patient may continue to bleed because she can no longer effectively clot. In
a hospital setting, this can be treated with infusions of platelets, fresh frozen plasma
and cryoprecipitate. If these products are unavailable, fresh
whole blood transfusion will give good results.
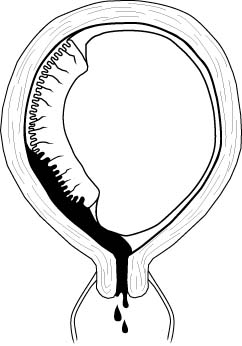
Placenta previa
Normally, the placenta is attached to the uterus in an
area remote from the cervix. Sometimes, the placenta is located in such a way that it
covers the cervix. This is called a placenta previa.
There are degrees of placenta previa:
-
A complete placenta previa means the entire cervix is covered. This
positioning makes it impossible for the fetus to pass through the birth canal without
causing maternal hemorrhage. This situation can only be resolved through cesarean section.
-
A marginal placenta previa means that only the margin or edge of the
placenta is covering the cervix. In this condition, it may be possible to achieve a
vaginal delivery if the maternal bleeding is not too great and the fetal head exerts
enough pressure on the placenta to push it out of the way and tamponade bleeding which may
occur.
Clinically, these patients present after 20 weeks with
painless vaginal bleeding, usually mild. This is in contrast to patients with
placental abruption, who usually experience significant pain and contractions.
An old rule of thumb is that the first bleed from
a placenta previa is not very heavy. For this reason, the first bleed is sometimes called
a "sentinel bleed."
Later episodes of bleeding can be very substantial and
very dangerous. This can lead to hypovolemic shock and maternal death. Because a pelvic exam may provoke further bleeding it is important to
avoid a vaginal or rectal examination in pregnant women during the second half of their
pregnancy unless you are certain there is no placenta previa.
Factors associated with an increased risk of placenta previa include:
The location of the placenta is best
established by ultrasound. If ultrasound is not available, one reliable
clinical method of ruling out placenta previa is to check for fetal head
engagement just above the pubic symphysis.
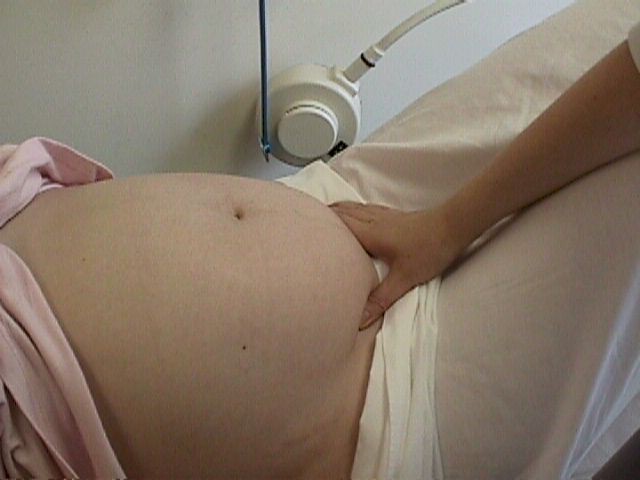 Using
a thumb and forefinger and pressing into the maternal abdomen, the fetal head
can be palpated. If it is deeply engaged in the pelvis, it is basically
impossible for a placenta previa to be present because there is not enough
room in the birth canal for both the fetal head and a placenta previa. An
x-ray of the pelvis (pelvimetry) can likewise rule out a placenta previa, but
only if the fetal head is deeply engaged. Otherwise, an x-ray will usually not
show the location of the placenta. Using
a thumb and forefinger and pressing into the maternal abdomen, the fetal head
can be palpated. If it is deeply engaged in the pelvis, it is basically
impossible for a placenta previa to be present because there is not enough
room in the birth canal for both the fetal head and a placenta previa. An
x-ray of the pelvis (pelvimetry) can likewise rule out a placenta previa, but
only if the fetal head is deeply engaged. Otherwise, an x-ray will usually not
show the location of the placenta.
Clinical
approach to third trimester bleeding
The clinical approach depends on the clinical situation. For
example:
-
A 3rd trimester patient who is hemorrhaging (500 cc with
continuing active bleed) bright red blood should go directly to the
operating room for a cesarean section to deliver her from the
placental abruption or placenta previa. While en route to the OR,
call for blood transfusions and labs to determine coagulopathy.
-
A patient at term with regular contractions and a small amount of
bloody mucous can be examined vaginally after confirming (through
ultrasound or clinical exam of the abdomen) that there is no
placenta previa.
-
Patients with bright red vaginal bleeding that is less than
hemorrhage should be carefully evaluated prior to performing a
pelvic exam. Ultrasound can be helpful in locating the placenta and
looking for retroplacental blood clot. Laboratory tests for
coagulopathy can be helpful. Hgb is useful, not to determine whether
to transfuse or not (that is a clinical, not laboratory decision),
but to indicate the margin of safety available to the clinician in
caring for this patient. Continuous electronic fetal monitoring is
important to determine the degree of tolerance the fetus has for
this bleeding and the extent to which uteroplacental circulation has
been disrupted. After ruling out a placenta previa, examine the
patient with a speculum to determine the source of the bleeding
(from the cervical os? from the surface of the cervix? from a
laceration of the vaginal wall? etc.)
Clinical management of
hemorrhagic shock
Shock is the generalized inadequate perfusion of the tissues of
the body. The cause of shock may be low cardiac output (cardiogenic shock), low
blood volume (hypovolemic shock), or other other etiologies (septic shock, toxic
shock).
The greater the blood loss, the worse the clinical problem and the
more dramatic the clinical findings. This has led to a number of systems
to categorize hemorrhage according to its severity. One such system is
shown here:
|
Class
I |
Class
II |
Class
III |
Class
IV |
Severity |
Compensated |
Mild |
Moderate |
Severe |
% Loss |
Up to
15% |
15-30% |
30-40% |
>40% |
Volume
Loss |
Up to
500 ml |
750-1500
ml |
1500-2000 |
>2000 |
Pulse |
<100 |
>100 |
>120 |
>140 |
BP |
Normal |
Orthostatic
change |
Mod.
Decrease |
Frank
Hypotension |
Respiratory
Rate |
Normal |
Mild
increase |
Tachypnea |
Marked
tachypnea, Respiratory failure |
Urine
(ml/hour) |
>30 |
20-30 |
5-20 |
None |
Symptoms |
Normal |
Agitated,
anxious |
Confused,
disoriented |
Lethargic,
unresponsive, Skin cold and clammy |
In real patients, there is often some overlap between classes, and
not all individuals within a class will show all of these signs and
symptoms.
Pregnancy itself can be a complicating factor. Following a normal
vaginal or cesarean delivery, maternal blood loss may average as much as
1,000 ml, or about 20% of the maternal blood volume. In a non-pregnant
adult, such a loss would likely provoke signs and symptoms of shock. But
in pregnant women, this loss is tolerated, often without any significant
symptoms, primarily because the mother's blood volume needs immediately
after delivery are significantly less than just before delivery. The
fetus and placenta (with all their metabolic needs) are gone from the
mother. The uterus is firmly contracted, reducing blood flow through it
and the eliminating the shunting effect of the intervillous space. The
maternal peripheral blood flow, previously widely dilated,
vasoconstricts, reducing the distensibility of her cardiovascular
system. Her pre-preganancy blood volume has increased by about 50% over
the course of pregnancy(30% increase in red cell mass) and so the loss
of 20% of her blood volume during delivery is generally tolerated very
well. Blood loss in excess of the normal loss, however, can be
problematic, as can any significant blood loss while she remains
pregnant. Unfortunately, the bleeding associated with placenta previa
and placental abruption both occur while she continues to have strong
metabolic needs of the fetus and placenta, significant cardiac output
directed to the uterus, and significant shunting through the
intervillous space.
 Management of hemorrhagic shock involves two processes, recognition
and treatment. Management of hemorrhagic shock involves two processes, recognition
and treatment.
Recognition requires being alert to the significance of any observed
bleeding and its context. Rapid loss of 500 cc of bright red blood
during a normal vaginal delivery would not attract clinical attention,
so long as it stopped as soon as the uterus firmly contracted. The same
500 cc loss from a woman not in labor would constitute hemorrhage. The
sudden appearance of 500 cc of bright red blood and clots in a woman in
active labor at 6 cm suggests both hemorrhage and a placental abruption.
Recognition is complicated by some of the routine procedures used
during labor. Many patients receive IV fluids during labor that may
partially compensate for active bleeding. It is not unusual for a woman
who is steadily bleeding, but who is receiving IV fluids, to have
reasonably normal vital signs up until she experiences cardiovascular
collapse.
Recognition involves watching maternal vital signs for the classical
signs of tachycardia, tachypnea, hypotension and oliguria, as well as
the symptoms of agitation, anxiety, confusion, disorientation and
lethargy. The second part of recognition is observing significant blood
loss, either at an unusual time, or in excess of the expected. Either
may trigger treatment for hemorrhage.
Treatment:
-
IV crystalloid. Up to two liters can effectively treat mild
degrees of hemorrhage. In more severe hemorrhage, it can stabilize
the patient long enough to get blood transfusions going. Some
physicians prefer a colloid solution (and there are arguments both
for and against this). Either should be effective in helping expand
the intravascular volume.
-
Oxygen. This won't help a lot because the hemoglobin is
already nearly 100% saturated with oxygen, but it helps enough that
it is worth doing.
-
Blood. The problem with shock is that not enough oxygen is
getting to the tissues. Expanding the blood volume with crystalloid
won't create any more red blood cells to carry oxygen to the
tissues. In cases of moderate to severe shock, blood transfusions
are needed.
-
Trendelenberg position. If blood is not immediately
available, placing the patient in a head-down position (ie, legs up
in the air, or Trendellenberg position) will make available 250 to
500 cc of blood that had been pooled in the lower extremeties.
-
Stop the bleeding. Do whatever needs to be done to stop the
bleeding. There is an old medical expression: "All bleeding
eventually stops." That's true.
Blood transfusion
The ideal material for use in hemorrhagic shock would be
disease-free, body-temperature, fresh, whole blood, with identical blood type
and major and minor blood groups. This has excellent oxygen-carrying capacity,
platelets, coagulation factors, volume and colloid, and would be totally
non-reactive within the victim's bloodstream. Unfortunately, this ideal material
does not exist (at least not when quickly needed), so we usually compromise,
using various blood products, depending on the needs of the patient.
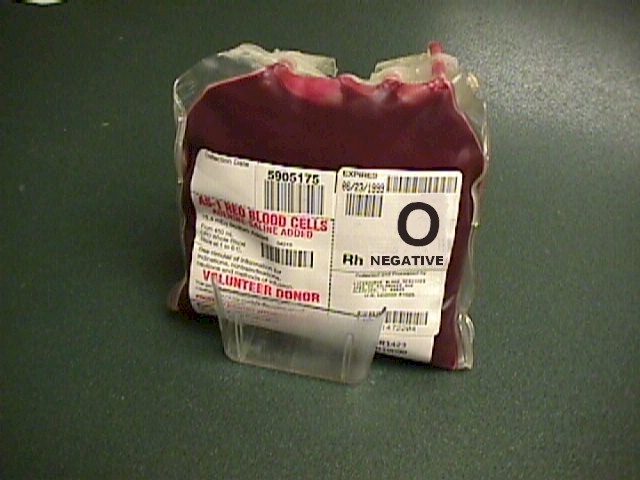
 In an extreme emergency, you can give the patient type O negative
packed RBCs from the blood bank. For someone whose blood type is unknown
and there is no time for cross-matching, this is the best approach. If
type O negative blood is not available, type O positive can be
substituted with good results, but there is a fair chance that the
patient (if Rh negative) will become sensitized against the Rh factor.
Still, that may be preferable to losing the patient because they weren't
transfused quickly enough. If the patient's blood type is known, you can
give type specific blood to her. If she is type B positive, you can give
type B positive blood to her. In either case, it is best to draw a plain
tube of blood just before giving the non-crossmatched blood to help in
figuring out her native blood type. In an extreme emergency, you can give the patient type O negative
packed RBCs from the blood bank. For someone whose blood type is unknown
and there is no time for cross-matching, this is the best approach. If
type O negative blood is not available, type O positive can be
substituted with good results, but there is a fair chance that the
patient (if Rh negative) will become sensitized against the Rh factor.
Still, that may be preferable to losing the patient because they weren't
transfused quickly enough. If the patient's blood type is known, you can
give type specific blood to her. If she is type B positive, you can give
type B positive blood to her. In either case, it is best to draw a plain
tube of blood just before giving the non-crossmatched blood to help in
figuring out her native blood type.
If you have more time, it is better to give cross-matched blood.
Cross-matched means the type (A, B, AB, or O) will match, and the major
factors (Rh, Kell, etc.) will match. There will undoubtedly be some
minor factors that do not match, and the degree of a match hinges, in
part, on the size of your blood bank and the available time.
Transfusions are usually with packed RBCs (following removal of much
of the serum, coagulation factors, platelets and fibrinogen). This works
well enough and is very effective at restoring oxygen carrying capacity.
Give enough units so the kidney function is normal (>30 cc/hour) and
the patient has no more than mild symptoms of hypovolemia. There is no
specific hemoglobin concentration that should be a target. The old
target of 10 gm dL of hemoglobin is too conservative, requiring more
blood transfusions than are really needed.
Following transfusion, the per-unit risk of developing:
-
Hepatitis C is about 1 in 3300
-
Hepatitis B is about 1 in 200,000
-
HIV is about 1 in 500,000
In some cases of severe hemorrhage, a coagulopathy develops as a
consequence of loss of platelets and/or clotting factors. This situation
can be determined through lab tests (PT, PTT, fibrinogen, platelet
count, bleeding time, and others), or clinically by observing multiple
bleeding sites from the mouth, gums, needle puncture sites or surgical
sites. A crude estimate may be obtained by taping a red-top tube of
blood to the wall. A clot should form within about 5 minutes. If it
doesn't, a coagulopathy of some form can be presumed. Coagulopathy can
be suspected in anyone with massive blood loss (requiring 4 or more
units of RBCs to correct).
Fresh frozen plasma (FFP) contains plasma proteins and clotting
factors. For patients who are actively bleeding and have a demonstrated
coagulopathy with prolonged PT and PTT, FFP will help restore hemostasis.
For those not actively bleeding, FFP is not often needed if the PT and
PTT are prolonged no more than 1.5 times normal. FFP need not be given
prophylactically with RBC transfusions in the absence of coagulopathy.
However, if the total blood volume has been replaced in an individual,
FFP is sometimes given.
If the only missing factor is fibrinogen (sometimes the case with
placental abruptions and often the case with long-standing missed
abortions), then cryoprecipitate can be given to replace the fibrinogen.
Cryoprecipitate also contains von Willebrand's factor and is useful in
treating bleeding patients with this particular deficiency.
Platelets are given if the platelet count falls below 50,000 and the
patient continues to bleed. In the absence of bleeding, platelet counts
between 10,000 and 50,000 are worrisome, but usually not an indication
for platelet transfusion. If the platelets fall below 10,000, the
patient should be transfused as the risk for CNS and GI bleeding rises
significantly. Pre-operatively, those with platelet counts below 50,000
may be transfused.
 Transfusion Without a Blood Bank Transfusion Without a Blood Bank
In civilian settings, banked blood is usually given. In many operational
settings, standard blood banking procedures may not
be applicable or available. In these cases, direct donor to victim transfusion
can be life-saving.
-
Use a donor with O negative blood ("Universal
Donor"). Don't try to match, for example, a B+ victim to a B+ donor. While the
accuracy of blood type records has improved, there is still a significant
inaccuracy rate (as high as 5%) in the medical record laboratory reports,
identification cards, and dog tags. If you try to match a B+ victim to a B+
donor (type-specific blood transfusion), you are twice taking a 5% risk of a
mismatch. It is safer to take that risk only once. If
the only available blood for a Rh negative victim is Rh positive blood, Rhogam
may be used, in very large doses of 25-30, full-size, 300 microgram ampoules,
IV, per unit of blood, to neutralize the Rh sensitizing effects of the Rh
positive blood.
-
Arrange IV tubing so that there is a
large-bore needle at each end. This is facilitated by use of a 3-way stopcock.
If this is not available, you can simply cut off the tubing at the end and
insert it into the hub of a needle. Sterile petroleum jelly can provide a seal
and the needle is held tightly to the IV tubing with adhesive tape.
-
Position the donor about 3 feet higher than
the victim. With the victim in a lower bunk, the donor would be in an upper
bunk. With the victim on the floor or on the deck, the donor would be on a cot
or packing crate.
-
Insert the IV into the donor and let the
blood flow downhill through the tube until it reaches the other end. Clamp the
tubing just long enough to insert the other end into the victims IV or vein.
-
Unclamp the tubing and allow time for about 1
unit (500 cc) of blood to flow into the victim. The exact amount of time
would depend on the caliber of the tubing and needle, length of the tubing,
height of the donor above the victim and doubtless other factors. In practice,
allow about 10 minutes, but be prepared to stop it earlier if the donor
becomes light-headed or dizzy.
-
Because fresh, whole blood has better
oxygen-carrying capacity than banked units of packed RBCs, and it is prewarmed,
and because it contains platelets, clotting factors and serum proteins, each
unit has about twice the clinical impact of a unit of packed cells from the
bank. If, based on your clinical experience, you believe a patient would
benefit from two units of PRBCs from a blood bank, they will generally do well
with a single unit of fresh, whole blood.
-
After the patient is transferred to a
definitive care facility, it will be easier for them to identify the true,
native blood type (major and minor blood groups) if they have a sample of
blood taken from the patient prior to any transfusions. If time permits and
the tactical situation allows for it, try to draw a single red-topped tube of
the victim's blood prior to transfusion that you can send along with the
MEDEVAC for use by blood banks further up the line.
|