 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
|
|
|
|
|
|
||||
|
||||||||||
|
|
|
|
|
|
| Recommendations and Reports |
|
|
|
|
|
|
| August 16, 2002 / 51(RR11);1-22 |
|
|
|
|
|
Prevention of Perinatal Group B Streptococcal DiseaseRevised Guidelines from CDCPrepared by The material in this report was prepared by the National Center for Infectious Diseases, James M. Hughes, M.D., Director; Division of Bacterial and Mycotic Diseases, Mitchell L. Cohen, M.D., Director. Summary Group B streptococcus (GBS) remains a leading cause of serious neonatal infection despite great progress in perinatal GBS disease prevention in the 1990s. In 1996, CDC, in collaboration with other agencies, published guidelines for the prevention of perinatal group B streptococcal disease (CDC. Prevention of perinatal group B streptococcal disease: a public health perspective. MMWR 1996;45[RR-7]:1--24). Data collected after the issuance of the 1996 guidelines prompted reevaluation of prevention strategies at a meeting of clinical and public health representatives in November 2001. This report replaces CDC's 1996 guidelines. The recommendations are based on available evidence and expert opinion where sufficient evidence was lacking. Although many of the recommendations in the 2002 guidelines are the same as those in 1996, they include some key changes:
Although universal screening for GBS colonization is anticipated to result in further reductions in the burden of GBS disease, the need to monitor for potential adverse consequences of intrapartum antibiotic use, such as emergence of bacterial antimicrobial resistance or increased incidence or severity of non-GBS neonatal pathogens, continues, and intrapartum antibiotics are still viewed as an interim strategy until GBS vaccines achieve licensure. IntroductionGroup B streptococcus (GBS) emerged as the leading infectious cause of neonatal morbidity and mortality in the United States in the 1970s (1--4). Initial case series reported case-fatality ratios as high as 50%. In the early 1980s, clinical trials demonstrated that administering antibiotics during labor to women at risk of transmitting GBS to their newborns could prevent invasive disease in the first week of life (i.e., early-onset disease) (5). As a result of the collaborative efforts of clinicians, researchers, professional organizations, parent advocacy groups, and the public health community in the 1990s, recommendations for intrapartum prophylaxis to prevent perinatal GBS disease were issued in 1996 by the American College of Obstetricians and Gynecologists (ACOG) (6) and CDC (7), and in 1997 by the American Academy of Pediatrics (8). Those guidelines recommended the use of one of two prevention methods, a risk-based approach or a culture-based screening approach. Providers using the risk-based method identify candidates for intrapartum chemoprophylaxis according to the presence of any of the following intrapartum risk factors associated with early-onset disease: delivering at <37 weeks' gestation, having an intrapartum temperature >100.4ºF (>38.0ºC), or rupture of membranes for >18 hours. The screening- based method recommends screening of all pregnant women for vaginal and rectal GBS colonization between 35 and 37 weeks' gestation. Colonized women are then offered intrapartum antibiotics at the time of labor. Under both strategies, women with GBS bacteriuria during their current pregnancy, or who previously gave birth to an infant with early-onset GBS disease are candidates for intrapartum antibiotic prophylaxis. Before active prevention was initiated, an estimated 7,500 cases of neonatal GBS disease occurred annually (9). Despite striking declines in disease incidence coinciding with increased prevention activities in the 1990s, GBS disease remains a leading infectious cause of morbidity and mortality among newborns in the United States (10,11). Moreover, since the release of the 1996 guidelines, new data are available to evaluate the effectiveness of the screening approach relative to the risk-based approach and to resolve some of the clinical challenges of implementing prevention. In light of these new data, in November 2001, CDC consulted with multiple partners to revise the 1996 guidelines for the prevention of perinatal group B streptococcal disease, using an evidence-based approach where possible and scientific opinion when sufficient data were lacking (Table 1). These updated guidelines replace CDC's 1996 guidelines. They are intended for the following groups: providers of prenatal, obstetric, and pediatric care; supporting microbiology laboratories, hospital administrators and managed care organizations; childbirth educators; public health authorities; and expectant parents and their advocates. Differences and similarities between current and previous guidelinesFollowing are major differences in the new guidelines:
Although important changes have been instituted, many recommendations remain the same:
BackgroundEarly Infancy and Pregnancy-Related InfectionsGBS causes severe invasive disease in young infants. The majority of infections in newborns occur within the first week of life and are designated early-onset disease. Late-onset infections occur in infants aged >1 week, with most infections evident in the first 3 months of life. Young infants with invasive GBS disease usually present with sepsis or pneumonia, and less often contract meningitis, osteomyelitis, or septic arthritis. The proportion of infants with meningitis is higher among those with late-onset infections. When neonatal infections caused by GBS appeared in the 1970s, as many as 50% of patients died. During the 1990s, the case-fatality ratio of early- and late-onset disease was 4% (10) because of advances in neonatal care. Intrauterine infection of the fetus results from ascending spread of GBS from the vagina of a colonized woman who is typically asymptomatic. Fetal aspiration of infected amniotic fluid can lead to stillbirth, neonatal pneumonia, or sepsis. Infants can also become infected with GBS during passage through the birth canal, although the majority of infants who are exposed to the organism through this route become colonized on skin or mucous membranes but remain asymptomatic. In pregnant women, GBS can cause clinical infections, but most women have no symptoms associated with genital tract colonization. Urinary tract infections caused by GBS complicate 2%--4% of pregnancies (12,13). During pregnancy or the postpartum period, women can contract amnionitis, endometritis, sepsis, or rarely, meningitis caused by GBS (14--19). Fatalities among women with pregnancy-associated GBS disease are extremely rare. GBS ColonizationThe gastrointestinal tract serves as the natural reservoir for GBS and is the likely source of vaginal colonization. Vaginal colonization is unusual in childhood but becomes more common in late adolescence (20). Approximately 10% to 30% of pregnant women are colonized with GBS in the vagina or rectum (21). GBS colonization can be transient, chronic, or intermittent. Maternal intrapartum GBS colonization is a major risk factor for early-onset disease in infants, and vertical transmission of GBS from mother to fetus primarily occurs after the onset of labor or membrane rupture. However, colonization early in pregnancy is not predictive of neonatal sepsis (22). Culture screening of both the vagina and rectum for GBS late in gestation during prenatal care can detect women who are likely to be colonized with GBS at the time of delivery and are thus at higher risk of perinatal transmission of the organism (23). Classic epidemiologic studies conducted during the 1980s revealed that women with prenatal GBS colonization were >25 times more likely than women with negative prenatal cultures to deliver infants with early-onset GBS disease (24). Researchers used prenatal cultures as the basis for identifying candidates for intrapartum antimicrobial chemoprophylaxis; clinical trials identified reductions in vertical transmission of the organism, as measured by infant colonization (25,26) or by protection against early-onset disease (5,27). Heavy colonization, defined as culture of GBS from direct plating rather than only from selective broth, is associated with higher risk for early-onset disease. GBS identified in clean-catch urine specimens is considered a surrogate for heavy maternal colonization and also is associated with a higher risk for early-onset GBS disease (12,13); it has been included among indications for intrapartum antibiotic prophylaxis. GBS Culture-Based Screening MethodsNumerous studies have documented that the accuracy of prenatal screening cultures in identifying intrapartum colonization status can be enhanced by careful attention to the timing of cultures, the anatomic sites swabbed, and the precise microbiologic methods used for culture and detection of organisms (Box 1). Collection of cultures between 35 and 37 weeks' gestation is recommended to improve the sensitivity and specificity of detection of women who remain colonized at the time of delivery (23,28). Swabbing both the lower vagina and rectum (i.e., through the anal sphincter) increases the yield substantially compared with sampling the cervix or sampling the vagina without also swabbing the rectum (29). Studies have indicated that when women in the outpatient clinic setting collect their own screening specimens, with appropriate instruction, GBS yield is similar to when specimens are collected by a health-care provider (30). Although swabbing both sites is recommended and use of two swabs can be justified, both swabs should be placed in a single broth culture medium because the site of isolation is not important for clinical management and laboratory costs can thereby be minimized. Because vaginal and rectal swabs are likely to yield diverse bacteria, use of selective enrichment broth is recommended (Box 1) to maximize the isolation of GBS and avoid overgrowth of other organisms. When direct agar plating is used instead of selective enrichment broth, as many as 50% of women who are GBS carriers have false-negative culture results (31). Additional Risk Factors for Perinatal GBS DiseaseIn addition to colonization with GBS, other factors increase the risk for early-onset disease. These include gestational age <37 completed weeks, longer duration of membrane rupture, intraamniotic infection, young maternal age, black race, Hispanic ethnicity, and low maternal levels of anticapsular antibody (32--37). In a 1985 report of predictors of early-onset disease (24), women with gestation <37 weeks, membrane rupture of >12 hours, or intrapartum temperature >99.5ºF (37.5ºC) had 6.5 times the risk of having an infant with early-onset GBS disease compared with women with none of those factors. Of note, women who had one of these risk factors but who had negative prenatal screening cultures were at relatively low risk for early-onset GBS disease (attack rate 0.9 per 1,000 births) compared with women who were colonized prenatally but had none of the risk factors (attack rate 5.1 per 1,000 births) (24). In a risk-based strategy promoted during the 1990s as an alternative to prenatal culture-based screening approaches, prematurity (gestation <37 weeks), intrapartum fever (temperature >100.4ºF or 38ºC), or duration of membrane rupture >18 hours were used as clinical indications for intrapartum prophylaxis. Previous delivery of an infant with invasive GBS disease may increase the risk of early-onset disease in subsequent deliveries (38,39), and intrapartum treatment of such women in subsequent pregnancies has been promoted. By contrast, colonization with GBS in a previous pregnancy is not considered an indication for intrapartum prophylaxis in subsequent pregnancies; rather, women require evaluation for prenatal colonization in each pregnancy. Because colonization is transient, the predictive value of culture-based screening is too low to be clinically useful when performed more than 5 weeks before delivery (28); thus, many women with GBS colonization during one pregnancy will no longer be colonized during subsequent pregnancies. Impact and Implementation of the 1996 GuidelinesDeclines in Perinatal GBS Disease Incidence in the Era of ChemoprophylaxisBefore the widespread use of intrapartum antibiotics, the incidence of invasive neonatal GBS disease ranged from 2 to 3 cases per 1,000 live births (9,40). Active, population-based surveillance in selected states in 1990, when GBS prevention was still rarely implemented, projected an incidence of 1.8 cases per 1,000 live births in the United States (early-onset disease: 1.5/1,000; late-onset: 0.35/1,000) (9). Coinciding with active prevention efforts in the 1990s, the incidence of early-onset disease declined by 70% to 0.5 cases per 1,000 live births in 1999 (Figure 1). Projections from active surveillance data for 1999 from the Active Bacterial Core surveillance/Emerging Infections Program Network (ABCs)(41) estimate that intrapartum antibiotics prevented nearly 4,500 early-onset cases and 225 deaths that year (10,11). Other countries that have adopted perinatal GBS disease prevention guidelines similar to the United States have seen comparable declines in early-onset disease incidence (42--44). Recent estimates of early-onset disease incidence in the United States suggest a slight increase in incidence from 1999 to 2000, consistent with a plateau in the impact of prevention efforts (Figure 1). The incidence of invasive GBS infections among pregnant women in the United States declined by 21% from 0.29 per 1,000 live births in 1993 to 0.23 in 1998 (10), suggesting that increased use of intrapartum antibiotics also prevented some cases of maternal GBS amnionitis and endometritis. In contrast, the rate of late-onset disease remained fairly constant throughout the 1990s (Figure 1). Although intrapartum chemoprophylaxis for women with heavy GBS colonization may prevent a portion of late-onset disease, the stable incidence of late-onset disease during a period when use of intrapartum antibiotics was increasing suggests that this intervention is not effective against late-onset disease. Implementation of Chemoprophylaxis Strategies After the Release of the 1996 GuidelinesDeclines in perinatal GBS disease incidence in the 1990s suggest that prevention strategies have been implemented successfully. Several studies have explored directly the challenges of implementation and extent of compliance with recommendations. Surveys of prenatal care providers in Connecticut and Minnesota in 1998 found that over 80% had a GBS prevention policy (Connecticut, 95%; Minnesota, 85%) (45). In Minnesota, family physicians were less likely to have a policy than were obstetrician/gynecologists and certified nurse midwives (45). A national survey of ACOG members in 2000 found that 98% of respondents had a GBS prevention policy; 75% of respondents reported using a version of the culture-based screening approach (46). Providers in all three surveys scored well on questions about their knowledge of the screening and risk-based strategies (45,46). In hospitals that established or revised policies for GBS prevention shortly after the release of the 1996 guidelines, rates of early-onset GBS disease declined by 1997 (47). By 1999, although only 63% of hospitals in a multistate survey of hospitals in the ABCs areas had a formal GBS prevention policy (48), having a hospital policy was no longer associated with changes in incidence of GBS disease, likely because a high proportion of individual practitioners had adopted policies by this time. Several studies of single institutions or health maintenance organizations have evaluated adherence of hospital personnel to GBS guidelines (Table 2). Among hospitals with a risk-based policy, intrapartum antibiotics were administered in 40%--80% of preterm deliveries or deliveries with prolonged rupture of membranes (Table 2) (49--53). Among hospitals with a culture-based screening policy, close to 90% of delivering women had documented GBS screening, and close to 90% of GBS-positive women received intrapartum antibiotics (Table 2) (42,51,54--59). Correct laboratory processing of culture specimens (Box 1) plays a critical role in successful implementation of the screening policy. A survey of clinical laboratories in selected counties of three states in 1997--1998 found that only a proportion of laboratories were using the recommended selective broth media to process GBS cultures (Georgia, 39% of laboratories; Minnesota, 42%; Connecticut, 62%), suggesting that this may be an area in need of improvement (31). Although surveys of practitioners and laboratories and reports from single hospitals help monitor implementation of GBS prevention guidelines, a recent CDC-sponsored review of labor and delivery records in selected counties of eight states in the ABCs areas in 1998 and 1999 sheds light on actual provider practices 2 to 3 years after the release of the 1996 guidelines (60). In this population, GBS screening was documented in 52% of deliveries, although this varied widely, from 24% in selected counties of Oregon to 70% in Maryland. Among screened women, 24% were GBS positive, consistent with carriage rates reported in earlier studies; 89% of GBS- positive women received intrapartum antibiotics. The median time of GBS culture collection was at 35.6 weeks' gestation, consistent with the recommendation of 35--37 weeks' gestation. Among unscreened women, 24% had at least one intrapartum risk factor; however, only 61% of women with at least one risk factor received intrapartum antibiotics. Preterm delivery (<37 weeks' gestation) was the most common indication for which intrapartum antibiotics were not administered. Thus, this multistate record review confirmed trends in adherence identified in reports from single hospitals (Table 2). Maximizing Prevention by ChemoprophylaxisEffectiveness of the Risk-Based Approach Versus the Screening ApproachDespite dramatic declines in GBS incidence in the United States in the 1990s, GBS remains a leading cause of newborn morbidity and mortality, resulting in an estimated 1,600 early-onset cases and 80 deaths annually. Although alternatives to intrapartum antibiotics such as a vaccine may become available in the future, intrapartum chemoprophylaxis remains the most effective available intervention against perinatal GBS disease. However, debate about the most effective strategy for identifying candidates for intrapartum chemoprophylaxis continues. When the 1996 guidelines were issued, data regarding the relative effectiveness of the risk-based and screening approaches were not available. Theoretical predictions based on population estimates of the proportion of early-onset GBS cases without obstetric risk factors (approximately 45% in the preprevention era [61]) suggested that the screening-based approach would lead to greater declines in disease incidence than the risk-based approach (61,62). However, because implementation of the risk-based approach has been viewed as simpler than the screening-based approach, which requires correct specimen collection at the prenatal clinic, appropriate laboratory processing, and timely reporting of results to delivery staff, the actual effectiveness of these strategies is unknown. Consequently, since 1996, both approaches have been recommended as equally acceptable pending further data (6--8). Although observational data are now available suggesting that each strategy can lead to reduced incidence of early-onset GBS disease (49,50,63--65), the strategies have not been directly compared by clinical trial because of the large sample size required. A series of single hospital analyses finding benefits of screening over the risk-based approach (51,56,59,66) were limited by sequential use of the strategies and inability to control for potential confounders. A recent CDC-sponsored multistate study provided the first large-scale direct comparison of the strategies (60). By incorporating population-based surveillance for early-onset GBS disease into a sample survey of a population of over 600,000 live births, this analysis found that the screening approach was >50% more effective than the risk-based approach at preventing perinatal GBS disease. The protective effect of the screening approach was robust and persisted after controlling for risk factors associated with early-onset GBS disease (e.g., preterm delivery, prolonged membrane rupture, young maternal age, black race). The benefit of screening stemmed from two main factors. First, by identifying GBS-colonized women who did not present with obstetric risk factors, screening reached more of the population at risk than did the risk-based approach. Among the cohort of screened women, 18% of all deliveries were to mothers who were colonized with GBS but did not have obstetric risk factors. The efficacy of intrapartum antibiotics in preventing early-onset GBS disease among infants in this cohort was close to 90%, suggesting that chemoprophylaxis of GBS-positive women without obstetric risk factors resulted in significant prevention of early-onset disease. Women who were GBS positive in the screening cohort were also more likely to receive intrapartum antibiotics than were women with obstetric risk factors in the risk cohort. Although improvements in implementation of the risk-based approach would lead to further decline in disease, this would not be as great as with universal screening (60). Finally, because the effectiveness of screening in this study was based on actual implementation of this strategy in clinical practice in 1998 and 1999, further improvements in screening implementation (e.g., improvements in specimen collection and the methods used for processing cultures) are expected to result in further benefits. Rationale for a Universal Prenatal Screening Strategy to Detect GBS StatusThe new availability of category II evidence (Table 1) for a large protective effect of prenatal GBS screening compared with the risk-based approach provides the foundation for a recommendation of universal prenatal GBS screening (Figure 2). Statewide prevention activities in some ABCs areas further demonstrate that culture-based screening can be successfully implemented in a variety of settings and institutions. For example, a health department-led survey of clinical laboratories in Connecticut followed by rapid feedback of survey results found that the proportion of laboratories in Connecticut using the correct media for processing GBS screening cultures increased from 62% in 1997 to 92% in 1998 (67) and 100% in 2000. Moreover, coinciding with an active prevention campaign launched by the state health department that advocated the screening-based approach, the incidence of early-onset GBS disease in Connecticut declined from 0.6 cases per 1,000 live births in 1996 (68) to 0.2 cases per 1,000 live births in 1999. From the standpoint of implementation, universal screening has two additional benefits over the dual recommendations of 1996. Communication of the public health messages associated with a single strategy is simpler than communicating and educating about multiple strategies. Additionally, screening has clear indicators that facilitate evaluation of implementation (e.g., documentation of GBS test, timing of test, rates of GBS positivity) (58) compared with the risk-based approach, in which evidence of prevention implementation cannot be assessed for approximately 75% of deliveries because they have no intrapartum risk factors. Cost-effectiveness analyses of the screening- and risk-based strategies (62,69--73) have indicated that although the initial costs associated with specimen collection and processing make the screening strategy more expensive than the risk-based approach, the overall cost savings due to disease prevention do not differ importantly between strategies. Additionally, multistate review of labor and delivery records in 1998 and 1999 suggests that perfect implementation of the screening- or risk-based strategies will result in a comparable proportion of deliveries in which women receive intrapartum antibiotic prophylaxis for GBS (24% for both strategies) (60,74). Thus, the strategies cannot be distinguished in terms of the proportion of deliveries that will be exposed to intrapartum antibiotics. Adverse Effects and Unintended Consequences of ChemoprophylaxisPotential adverse or unintended effects of GBS prevention efforts that have raised concern include allergic or anaphylactic reactions to agents used for intrapartum antibiotic prophylaxis, emergence of GBS strains resistant to standard therapies, and increasing incidence of serious neonatal infections caused by pathogens other than GBS, including antimicrobial-resistant strains. Because of the increasing emergence of bacterial resistance to antimicrobial agents in both nosocomial and community settings, assessment of the impact and continued effectiveness of interventions based on antimicrobial prophylaxis is critical. Antibiotic Allergies Including AnaphylaxisAnaphylaxis associated with GBS chemoprophylaxis occurs but is sufficiently rare that any morbidity associated with anaphylaxis is greatly offset by reductions in the incidence of maternal and neonatal invasive GBS disease. Anaphylaxis-related mortality is likely to be a rare event since women receiving intrapartum antibiotics will be in hospital settings where rapid intervention is readily available. Estimates of the rate of anaphylaxis caused by penicillin range from 4/10,000 to 4/100,000 recipients. Additionally, as many as 10% of the adult population have less severe allergic reactions to penicillin (75). Anaphylaxis associated with GBS prophylaxis was reported in the early 1990s (76); since the release of the 1996 guidelines, an additional report of a nonfatal case of anaphylaxis associated with GBS chemoprophylaxis has been published (77). In a CDC multistate sample of over 5,000 live births, a single, nonfatal anaphylactic reaction was noted among the 27% of deliveries in which intrapartum antibiotics were administered (60). In that case, a single dose of penicillin was administered approximately 4 hours before a preterm cesarean delivery, and an anaphylactic reaction occurred shortly after the mother received a single dose of a cephalosporin following umbilical cord clamping. Resistance in GBSGBS isolates with confirmed resistance to penicillin or ampicillin have not been observed to date (78--83). Penicillin remains the agent of choice for intrapartum antibiotic prophylaxis. Ampicillin is an acceptable alternative, but penicillin is preferred because it has a narrower spectrum of antimicrobial activity and may be less likely to select for resistant organisms. The efficacy of both penicillin (27) and ampicillin (5) as intrapartum agents for the prevention of early-onset neonatal GBS disease has been demonstrated in clinical trials. Although the intramuscular route of administration for penicillin has been evaluated (25), intravenous administration is the only route of administration recommended for intra-partum chemoprophylaxis to prevent perinatal GBS disease, regardless of the antimicrobial agent used, because of the higher intraamniotic concentrations achieved with this method. In contrast, the proportions of GBS isolates with in vitro resistance to clindamycin and erythromycin have increased since 1996. The prevalence of resistance among invasive GBS isolates in the United States and Canada ranged from 7% to 25% for erythromycin and from 3% to 15% for clindamycin in reports published between 1998 and 2001(79--81,84). Resistance to erythromycin is frequently but not always associated with clindamycin resistance. Resistance of GBS isolates to cefoxitin, a second-generation cephalosporin sometimes used as a component of broad-spectrum coverage for chorioamnionitis, has also been reported (85); cefoxitin resistance has similarly been observed among invasive GBS isolates collected from 1996 to 2000 as part of CDC's active surveillance. Whether in vitro resistance of GBS has direct clinical implications remains unclear (86). Despite emerging resistance to some drug classes, minimum inhibitory concentrations of cefazolin, a first-generation cephalosporin available in an intravenous formulation, were low (<0.5 µg/ml) among a sample of invasive U.S. isolates from 1996 to 2000 (87), suggesting that GBS isolates are currently susceptible to this agent. Although NCCLS guidelines do not specify susceptibility breakpoints for cefazolin, they recommend that all isolates susceptible to penicillin be considered susceptible to cefazolin (88). In light of the increasing prevalence of resistance to clindamycin, erythromycin, or both, recommended strategies for providing intrapartum antibiotic prophylaxis to penicillin-allergic women are updated (Box 2). Because the efficacy of recommended alternatives to penicillin or ampicillin has not been measured in controlled trials, and because some of the recommended alternatives have a broad spectrum of activity and may be more complicated and costly to administer, verification of a reported history of penicillin allergy is important. Patients with reported penicillin allergy should then be assessed to determine their risk for anaphylaxis. Persons at high risk for anaphylaxis are those who have had immediate hypersensitivity reactions to penicillin (e.g., anaphylaxis, angioedema, or urticaria) or who have a history of asthma or other conditions that would make anaphylaxis more dangerous (89,90). An estimated 10% of persons with penicillin allergy also have immediate hypersensitivity reactions to cephalosporins (90). Among penicillin-allergic women not at high risk for anaphylaxis, cefazolin, because of its narrow spectrum of activity and ability to achieve high intraamniotic concentrations, is the agent of choice for intrapartum chemoprophylaxis. For penicillin-allergic women at high risk for anaphylaxis, testing of GBS isolates from prenatal screening for susceptibility to clindamycin and erythromycin is recommended if feasible (Box 1). One of these agents should be employed for intrapartum GBS prophylaxis if the screening isolate is susceptible to both agents. Vancomycin should be reserved for penicillin-allergic women at high risk for beta-lactam anaphylaxis when clindamycin or erythromycin are not options because of in vitro resistance or unknown susceptibility of a prenatal isolate. Vancomycin use is generally restricted because of emerging vancomycin resistance among some gram-positive organisms (e.g., vancomycin-resistant enterococcus and vancomycin-resistant Staphylococcus aureus). An estimated 13.8 million hospitalized patients received vancomycin therapy in 1998 (91). If penicillin allergy occurs in approximately 10% of adults, and 25% of parturients are colonized with GBS prenatally, approximately 100,000 of the 4 million annual deliveries would require prophylaxis with vancomycin in the absence of clindamycin and erythromycin susceptibility testing of GBS prenatal isolates. This represents a 7% increase in the number of patients exposed to vancomycin. The total grams of vancomycin used annually would increase by less than 1% if all penicillin-allergic colonized women received vancomycin prophylaxis. Increased Incidence or Resistance in Non-GBS PathogensDecreases in the incidence of early-onset GBS sepsis have not usually been accompanied by increases in incidence of early-onset sepsis caused by other pathogens, including those that are antibiotic resistant. Most studies, including population-based multicenter studies, have found stable (59,92,93) or decreasing (43) rates of non-GBS early-onset sepsis during a period of increasing use of intrapartum antibiotic prophylaxis for GBS (Table 3). This is true both for overall non-GBS sepsis and for neonatal sepsis caused by Escherichia coli, the second leading bacterial cause of neonatal sepsis after GBS (93,94). Some single hospital studies have found increased rates or case counts of neonatal sepsis caused by E. coli, gram-negative organisms in general, or ampicillin-resistant pathogens (64,94,95), but these increases appear to be limited to preterm or low-birth-weight infants. An increasing proportion of E. coli neonatal sepsis cases caused by ampicillin-resistant organisms was observed in two studies (92,94), but again was limited to preterm or low-birth-weight infants. Furthermore, the proportion of community-acquired E. coli infections that are ampicillin resistant has been increasing (96), suggesting that trends in antimicrobial resistance should not be attributed to GBS prophylaxis. An association between intrapartum antibiotic exposure and ampicillin resistance in cases of E. coli or other non-GBS early-onset sepsis has been observed in several studies (36,94,95, 97,98). These reports established that infections caused by antibiotic-resistant organisms were more frequently preceded by antibiotic use than were infections caused by susceptible organisms, and that more doses or longer duration of anti-biotics before delivery increased the chance that a neonatal infection, if it occurred, would be caused by an antibiotic-resistant organism. These studies, however, were not designed to assess whether intrapartum antibiotic use increased the rate of antibiotic-resistant infections. Moreover, findings from these studies are consistent with intrapartum antibiotics inducing resistance among initially susceptible organisms, but also with intrapartum antibiotics preventing antibiotic-susceptible infections and having no impact on antibiotic-resistant infections, resulting in a net decrease in the total rate of infection. The reported increases in antibiotic-resistant early-onset infections in a few studies are not of sufficient magnitude to outweigh the benefits of intrapartum antibiotic prophylaxis to prevent perinatal GBS disease. However, to assure early detection of increases in the rate of disease or deaths caused by organisms other than GBS, continued surveillance of neonatal sepsis caused by organisms other than GBS is needed. Clinical ChallengesGBS Bacteriuria During PregnancyThe presence of GBS bacteriuria in any concentration in a pregnant woman is a marker for heavy genital tract colonization. Therefore, women with any quantity of GBS bacteriuria during pregnancy should receive intrapartum chemoprophylaxis. Vaginal and rectal screening at 35--37 weeks is not necessary for these women. GBS can cause both symptomatic and asymptomatic urinary tract infections, which should be diagnosed and treated according to current standards of care for urinary tract infections in pregnancy. Women with GBS urinary tract infections during pregnancy should receive appropriate treatment at the time of diagnosis as well as intrapartum GBS prophylaxis. Laboratory personnel should report any presence of GBS bacteriuria in specimens obtained from pregnant women. For this to occur, labeling of urine specimens to indicate that they were obtained from a pregnant woman is imperative. Planned Cesarean DeliveryBecause GBS can cross intact amniotic membranes, a cesarean delivery does not prevent mother-to-child transmission of GBS. Moreover, because cesarean delivery itself is associated with health risks for mother and newborn, GBS colonization of the mother is not an indication for cesarean delivery, and cesarean delivery should not be used as an alternative to intrapartum antibiotic prophylaxis for GBS prevention. However, although a risk does exist for transmission of GBS from a colonized mother to her infant during a planned cesarean delivery performed before onset of labor in a woman with intact amniotic membranes, it is extremely low, based on a retrospective study at a single hospital (99) and a review of CDC active, population-based surveillance data from the 1990s. Thus, in this specific circumstance, in which the risk for disease is extremely low, the individual risks to a mother and her infant from receiving intrapartum antibiotic prophylaxis may balance or outweigh the benefits. Intrapartum antibiotic prophylaxis to prevent perinatal GBS disease is, therefore, not recommended as a routine practice for women undergoing planned cesarean deliveries in the absence of labor or amniotic membrane rupture, regardless of the GBS colonization status of the mother. Patients expected to undergo planned cesarean deliveries should nonetheless still undergo routine vaginal and rectal screening for GBS at 35--37 weeks because onset of labor or rupture of membranes may occur before the planned cesarean delivery. In rare situations in which patients or providers opt for intrapartum prophylaxis before planned cesarean deliveries, administration of antibiotics at the time of incision rather than at least 4 hours before delivery may be reasonable (100). Threatened Preterm DeliveryBecause preterm (at <37 weeks' gestation) delivery is an important risk factor for early-onset GBS disease, and because timing of delivery can be difficult to assess, management of intrapartum prophylaxis for women with threatened preterm delivery can be challenging. Assessing the need for intrapartum prophylaxis for these women can also be difficult because GBS screening is recommended at 35 to 37 weeks' gestation, and culture results are not always available when labor or rupture of membranes occur preterm. A suggested approach to GBS chemoprophylaxis in the context of threatened preterm delivery is outlined (Figure 3). Because insufficient data are available to suggest a single course of management, other management strategies developed by individual physicians or institutions may be appropriate alternatives. The algorithm suggests that if GBS screening culture results from the current pregnancy are not available and if onset of labor or rupture of membranes occurs before 37 weeks' gestation with a substantial risk for preterm delivery (as assessed by the woman's health-care provider), intrapartum antibiotic prophylaxis for GBS should be provided pending culture results. For women not yet screened for GBS, a vaginal and rectal specimen for GBS culture should be obtained if time permits. If a negative culture result within the previous 4 weeks is on record, or if the clinician determines that labor can be successfully arrested and preterm delivery averted, antibiotics for GBS prophylaxis should not be initiated. Because recent clinical trials suggest that antibiotics administered during pregnancy may be associated with adverse neonatal outcomes, such as necrotizing enterocolitis or increased need for supplementary oxygen, without evident benefit for preterm labor or preterm premature rupture of membranes (101,102), antibiotics should be reserved for instances in which a significant risk for preterm delivery is present. No data are available on which to recommend a specific duration of antibiotic administration for GBS-positive women with threatened preterm delivery when delivery is successfully postponed. Management strategies based on scientific opinion have been proposed (100); without further data, the management approach is left to the discretion of the individual provider. Regardless of management strategy chosen, these women should also receive intrapartum antibiotic chemoprophylaxis for GBS when labor likely to proceed to delivery occurs or recurs. Previous data (28) suggest that the accuracy of GBS screening cultures in predicting colonization status at delivery is greatest if the cultures are collected within 5 weeks of delivery. Therefore, if a woman is screened early for GBS because of threatened preterm delivery but does not deliver within 4 weeks, she should be screened again for GBS colonization and managed according to the result of the repeated screening culture (Figure 3). Obstetric Procedures for GBS-Colonized WomenQuestions have arisen regarding whether certain obstetric procedures, such as digital vaginal examinations, intrauterine fetal monitoring, and membrane stripping or sweeping to hasten the onset of labor, should be performed on GBS-colonized women. Asymptomatic GBS colonization is not an indication to perform any of these procedures. When such procedures are indicated for other reasons, evidence is currently not sufficient to recommend that particular procedures should be avoided because of increased risk of peripartum or perinatal infection. Although some obstetric procedures (frequent vaginal examinations after onset of labor or membrane rupture [17,36,103--105], intrauterine fetal monitoring [104,106,107], and mechanical cervical ripening devices [108]) have been significantly associated with peripartum or perinatal infectious outcomes, most studies to date have been limited by an inability to randomly allocate women to treatment groups and have yielded conflicting results. Moreover, because many studies were performed before GBS prevention was widely implemented, GBS colonization status was often not known and intrapartum chemoprophylaxis was less common. A meta-analysis of available studies examining the use of membrane stripping among women of undetermined GBS colonization status (109) found no significant increases in overall peripartum or perinatal infection rates among women who underwent this procedure and their infants compared with those who did not. Management of Newborns Exposed to Intrapartum ProphylaxisOn the basis of information available since the publication of the 1996 guidelines, a modified approach for empiric management of newborns born to women who receive intrapartum antibiotics to prevent early-onset GBS disease or to treat suspected chorioamnionitis is provided (Figure 4). Variations in the algorithm that incorporate individual circumstances or institutional preferences may be appropriate. The modified approach contains the following changes:
Investigations since 1996 lend additional support to several components of the algorithm. A retrospective study of over 250,000 live births (115) found that administration of intrapartum antibiotic prophylaxis did not change the clinical spectrum of neonatal illness or delay the onset of clinical signs among infants who contracted GBS disease despite prophylaxis. Thus, the algorithm targets infants born to mothers with suspected chorioamnionitis and infants with signs of sepsis for full diagnostic evaluation and empiric therapy. Also, new evidence indicates that 4 or more hours of intrapartum ampicillin or penicillin administered according to recommended dosing intervals (Box 2) significantly reduces vertical transmission of GBS (116) and risk of early-onset GBS disease (65). Thus, although the American Academy of Pediatrics 1997 guidelines suggested 2 or more doses as a threshold for prophylaxis adequacy for infants >35 weeks' gestation (8), the revised algorithm continues to use >4 hours, administered according to recommended dosing intervals, as the benchmark for optimal prevention of early-onset GBS disease. Moreover, a review of pregnancies at a West Coast health maintenance organization using the GBS culture-based screening strategy found that among women who received intrapartum antibiotic prophylaxis, 50% received prophylaxis at least 4 hours before delivery, whereas only 14% received at least 2 doses of intrapartum antibiotics (58); this indicates that duration of prophylaxis is a more practical target than number of doses, in addition to being associated with efficacy. One objective of developing an algorithm for management of newborns was to minimize unnecessary evaluation and antimicrobial treatment of infants whose mothers received intrapartum prophylaxis. Although early provider surveys indicated that pediatricians and neonatologists were more likely to conduct diagnostic evaluations and initiate empiric anti-biotics for an infant whose mother received intrapartum antibiotic prophylaxis (117--119), more recent data indicate that implementation of GBS prevention strategies has not resulted in increased use of health services for neonates (120), and in some circumstances, when GBS prophylaxis increased a decrease occurred in the proportion of neonates who received laboratory evaluations (58). Intrapartum antibiotic prophylaxis is the method of choice for preventing neonatal early-onset GBS disease. In the event that intrapartum antibiotics are not given despite an indication (e.g., delivery occurred precipitously before antibiotics could be administered to a GBS-positive woman), sufficient data are not available on which to recommend a single management strategy for the newborn. Some centers provide intramuscular penicillin to asymptomatic infants within 1 hour of birth, based on results of observational studies showing declines in early-onset GBS disease coincident with a policy of universal administration of intramuscular penicillin to all newborns (121). Future Prevention TechnologyRapid Tests to Detect GBS Colonization StatusRapid tests for detection of GBS colonization at the time of onset of labor or rupture of amniotic membranes might obviate the need for prenatal culture-based screening if their sensitivity and specificity are comparable to culture in selective broth media and they yield results rapidly enough to permit administration of adequate intrapartum antibiotic prophylaxis to women detected as carriers. Currently available rapid tests detect GBS antigen from swab specimens. These tests are insufficiently sensitive to detect light colonization, and therefore are not adequate to replace culture-based prenatal screening (122,123) or to use in place of the risk-based approach when culture results are unknown at the time of labor. An adequate rapid intrapartum test must be as sensitive as culture (minimally 85% compared with culture of vaginal and rectal swabs inoculated into selective broth media), rapid so that results are available to clinicians in time for antibiotics to be given before delivery, and convenient for integration into routine laboratory use. Even a highly sensitive rapid detection test would not be adequate if results were not available to clinicians 24 hours a day, 7 days a week. Alternatives to culturing vaginal and rectal swab specimens at 35--37 weeks' gestation using recommended procedures should be validated to show sensitivity similar to recommended culture methods. A rapid intrapartum test possessing the attributes described above offers the advantage of ascertaining GBS colonization status before delivery among women who have had no pre-natal care. Although such tests might initially be introduced selectively in certain facilities with sufficient demand and capability, a general recommendation for their use would require the capacity for effective implementation in a wide range of hospital settings. Drawbacks of rapid tests include delays in administration of intrapartum antibiotic prophylaxis while test results are pending and lack of an isolate for susceptibility testing, which is of particular concern for penicillin-allergic women. Additionally, until rapid tests are universally used, missed opportunities for GBS screening may occur among women who receive prenatal care at institutions relying on intrapartum rapid tests but who deliver at institutions where such tests are not yet available. In a study of 112 pregnant women at an academic hospital in Quebec, a new, not yet commercially available fluorogenic polymerase chain reaction assay was 97% sensitive and 100% specific when compared with vaginal and rectal cultures collected at admission for delivery. Test results in this study were available within 45 minutes of specimen collection (124). Further studies are needed to determine whether this type of test can be adapted for use outside the research setting. If appropriate techniques for rapid detection of GBS become commercially available, they may be integrated into the currently recommended screening strategy. Vaccines To Prevent GBS DiseaseImproved use of intrapartum antimicrobial prophylaxis has resulted in a substantial reduction in early-onset GBS disease, but it is unlikely to prevent most late-onset neonatal infections, GBS-related stillbirths, or prematurity, and does not address GBS disease in nonpregnant adults. Immunization of women during or before pregnancy could prevent peripartum maternal disease and protect infants from perinatally acquired infection by transplacental transfer of protective IgG antibodies (125,126). This would eliminate the need for prenatal GBS screening and intrapartum antimicrobial prophylaxis, along with associated costs and concerns regarding the potential adverse effects of intrapartum antibiotic use discussed previously. Serotype-specific antibodies to GBS capsular polysaccharide, although rare in populations of unvaccinated women, have been shown to protect against disease (32,127). Phase 1 and 2 clinical trials among healthy, nonpregnant adults of monovalent protein-conjugate vaccines containing capsular polysaccharide antigens of GBS disease-associated serotypes have shown these vaccines to be well tolerated and immunogenic (128--130). One challenge of demonstrating vaccine efficacy in preventing early-onset GBS disease is that the sample size required for clinical trials may be prohibitively large. Identification of surrogate immunologic measures of clinical efficacy may thus be important (131,132). Surrogate information on clinical vaccine efficacy may also be gained by measuring the impact of multivalent conjugate vaccines on vaginal GBS colonization (132,133). Anticipated difficulties in making vaccine available to pregnant women have resulted in consideration of other target populations for vaccine administration, including adolescent girls (134), women of childbearing age, and infants (135). The duration of protection that could be afforded by vaccination is unknown; one or more booster doses might be required, potentially complicating vaccine delivery. Shifts in the GBS serotypes causing disease have provided an additional challenge to vaccine development (133) and may necessitate modification of vaccine serotype composition over time. Research Priorities and Tools To Aid PreventionTechnological advances that aid the implementation of a universal screening strategy will further prevention efforts. In addition to development of reliable rapid tests that can be performed in a wide range of labor and delivery settings, methods of simplifying prenatal culture procedures, e.g., the development of media with a reliable color indicator to signal presence of GBS, might improve accuracy of prenatal culture results and facilitate prenatal culture processing at clinical laboratories with limited technical capacity. Media that have been developed for this purpose, such as Granada (136,137) or GBS medium (138), should be further evaluated to determine if sensitivity and specificity are comparable to recommended methods, which consist of culture in selective broth media followed by GBS-specific identification. Although universal prenatal GBS culture-based screening is likely to result in substantial further declines in the incidence of early-onset disease, intrapartum chemoprophylaxis is not a permanent or comprehensive strategy for GBS disease prevention. Because vaccines under development hold promise to prevent a larger portion of the burden of GBS disease with a simpler and sustainable intervention, further work on GBS vaccine development and support of phase 3 clinical trials are warranted (139). Until a safe, effective, and economical vaccine achieves licensure, it will be important to continue to monitor for potential adverse effects of chemoprophylaxis, with an emphasis on tracking key sentinel events signaling a need for revision of the guidelines. Such sentinel events include the emergence of penicillin resistance among GBS, which to date has not been detected, and an increase in the incidence of disease or deaths due to neonatal pathogens other than GBS that offsets the burden of early-onset disease prevented by chemoprophylaxis. Monitoring for the latter will require long-term surveillance of a large population of term and preterm births (140). Because GBS carriage is common among delivering women in the United States, continued surveillance for GBS disease and evaluation of prevention implementation remains important to minimize missed opportunities for prevention. States are encouraged to monitor incidence of GBS disease, to promote activities that enhance perinatal GBS disease prevention and education, and to assess progress toward national objectives for disease reduction, such as Healthy People 2010, which sets a target of reducing the incidence of early-onset GBS disease in all racial and ethnic groups to 0.5 cases per 1,000 live births (141). Practical tools to assist with monitoring for missed opportunities for perinatal GBS prevention within hospitals have been published (142); additional prevention information and tools for providers, patients and clinical microbiologists are available at http://www.cdc.gov/groupbstrep, http://www.acog.org/, http://sales.acog.com/, http://www.aap.org/, and http://www.health.state.mn.us/divs/dpc/ades/invbact/strepb.htm. RecommendationsThe following updated recommendations for the prevention of GBS disease are based on critical appraisal of multistate population-based observational data and several studies from individual institutions that have been completed since publication of previous CDC (7), ACOG (6), and AAP (8) recommendations. They replace previous recommendations from CDC. The strength (indicated by a letter) and quality (indicated by a roman numeral) of evidence supporting each recommendation are shown in parentheses, according to the evidence-based rating system outlined in Table 1. Obstetric-care practitioners, in conjunction with supporting laboratories and labor and delivery facilities, should adopt the following strategy for the prevention of perinatal GBS disease based on prenatal screening for GBS colonization. The risk-based approach is no longer an acceptable alternative except for circumstances in which screening results are not available before delivery (AII).
Before full implementation of this strategy can be expected in all health-care settings, all members of the health-care team will need to improve protocols for isolation and reporting of GBS culture results, to improve information management to ensure communication of screening results, and to educate medical and nursing staff responsible for prenatal and intrapartum care. Within institutions, such efforts may take several months. Even with ideal implementation, cases of early-onset GBS disease will continue to occur. Tools to help promote prevention and educate parents of infants with early-onset GBS disease are available at http://www.cdc.gov/groupbstrep. Additional tools available to assist with prevention implementation are available at http://www.acog.org/, http://sales.acog.com/, http://www.aap.org/ and http://http://www.health.state.mn.us/divs/dpc/ades/invbact/strepb.htm Multiple copies of educational materials published by CDC are available at the Public Health Foundation, 1220 L St., NW Suite 350, Washington, DC 20005, telephone 877-252-1200, or online at http://www.phf.org/. References
Consultants Kathryn Arnold, M.D., Georgia Division of Public Health, Atlanta, Georgia; Carol Baker, M.D., American Academy of Pediatrics/ Committee on Infectious Diseases, Elk Grove Village, Illinois; Gina Burns, M.S., Group B Strep Association, Chapel Hill, North Carolina; Richard Facklam, Ph.D., CDC, Atlanta, Georgia; Monica Farley, M.D., Infectious Diseases Society of America, Alexandria, Virginia; Theodore G. Ganiats, M.D., American Academy of Family Physicians, Leawood, Kansas; Ronald Gibbs, M.D., Infectious Diseases Society of Obstetricians and Gynecologists, Washington, D.C.; Paul Heath, M.D., U.K. GBS Prevention Working Group, London, England; James H. Jorgensen, Ph.D., Health Science Center at San Antonio, University of Texas, San Antonio, Texas; William Kanto, M.D., American Academy of Pediatrics/Committee on Fetus and Newborn, Elk Grove Village, Illinois; Shelene Keith, Jesse Cause Foundation--Saving the Babies from Group B Strep, Port Huenme, California; Tekoa King, M.P.H., American College of Nurse Midwives, Washington, D.C.; Feng Ying Lin, M.D., National Institute of Child Health and Development, National Institutes of Health, Bethesda, Maryland; Ruth Lynfield, M.D., Minnesota Department of Health, Minneapolis, Minnesota; Martin McCaffrey, M.D., Naval Medical Center, San Diego, California; Elliot Philipson, M.D., Cleveland Clinic, Cleveland, Ohio; Lisa Porter, M.Ed., Jacksonville, Florida; Laura Riley, M.D., American College of Obstetricians and Gynecologists, Washington, D.C.; Donna Russell, M.S., Washington Department of Health, Renton, Washington; Pam Sims, Pharm. D., Samford University, Birmingham, Alabama; Carol A. Spiegel, Ph.D., University of Wisconsin, Madison, Wisconsin; Barbara Stoll, M.D., Emory University School of Medicine, Atlanta, Georgia; Beth H. Stover, Healthcare Infection Control Practices Advisory Committee, CDC, Atlanta, Georgia; Cynthia Whitney, M.D., CDC, Atlanta, Georgia; Michael K. Yancey, M.D., Tripler Army Medical Center, Honolulu, Hawaii; Elizabeth Zell, M.Stat., CDC, Atlanta, Georgia Table 1 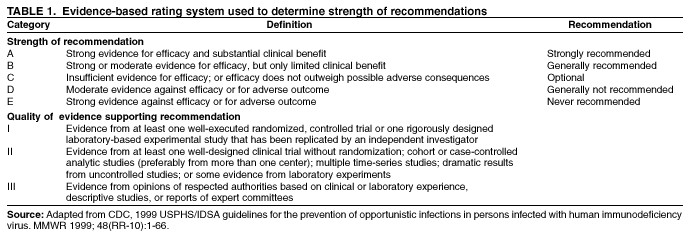 Return to top. Figure 1 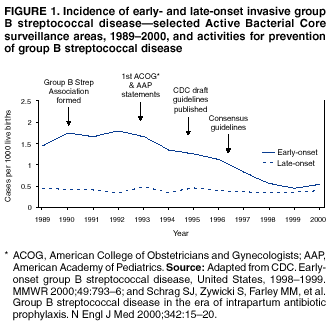 Return to top. Box 1  Return to top. Table 2 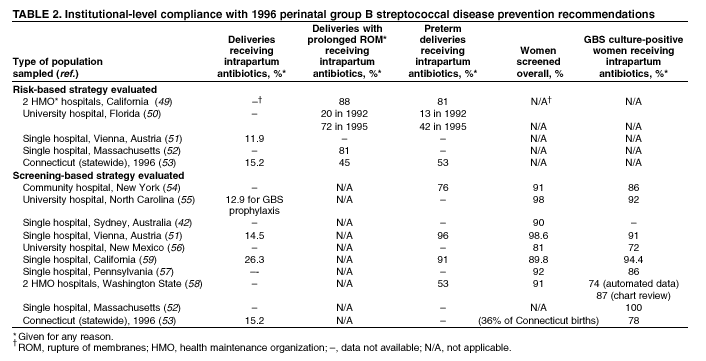 Return to top. Figure 2 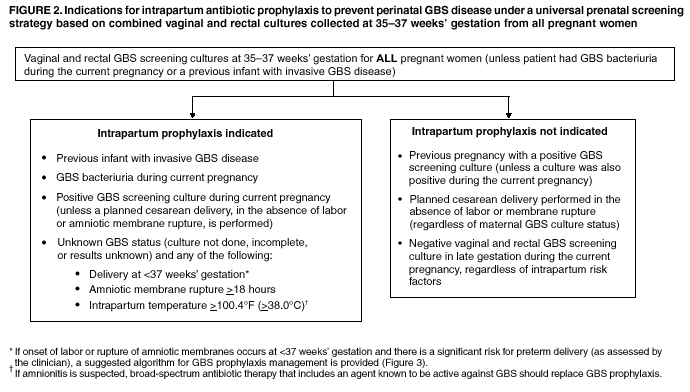 Return to top. Box 2 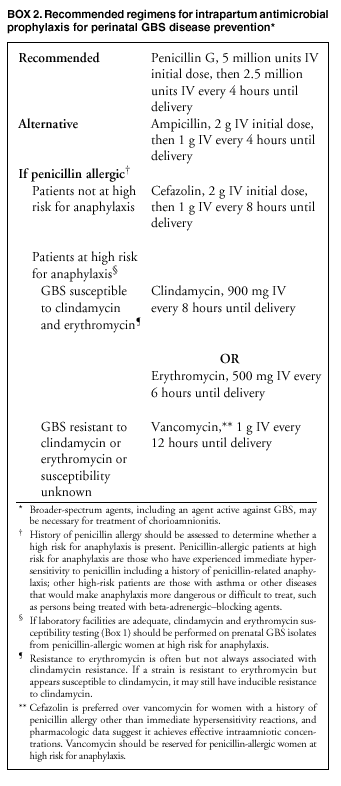 Return to top. Table 3 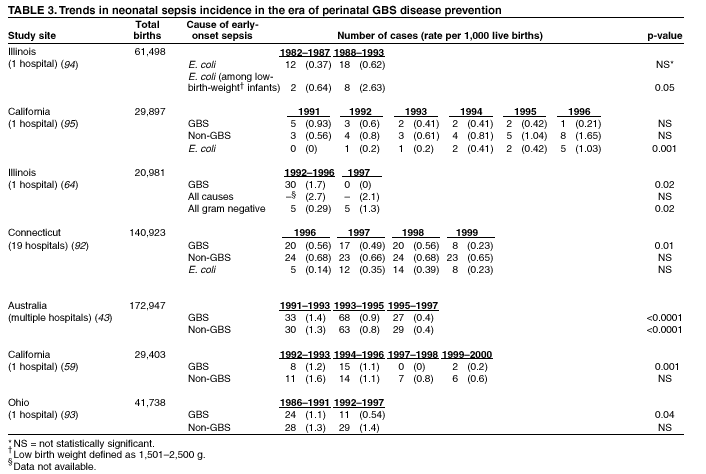 Return to top. Figure 3 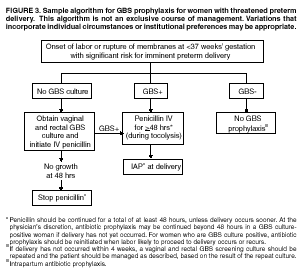 Return to top. Figure 4 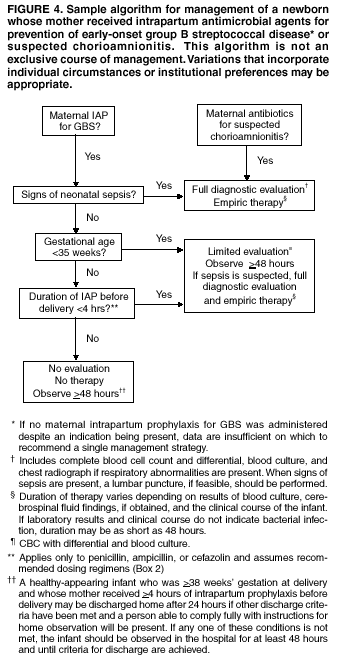 Return to top.
All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 8/6/2002 |
||||
This page last reviewed 8/6/2002 |